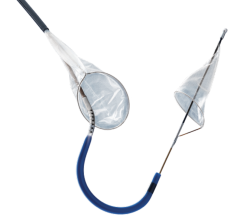
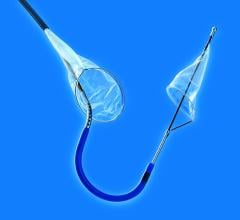
Boston Scientific launched its FilterWire EZ Embolic Protection System in a new 2.25 – 3.5 mm size, designed to contain and remove embolic material that may be dislodged during an interventional saphenous vein graft (SVG) procedure or material that travels into the microvasculature where it could pose an increased risk for a heart attack.
Recently revised treatment guidelines by the American College of Cardiology have recommended the use of embolic protection devices when treating patients with SVG disease, and a filter-based solution smaller than 3.0 mm has not available for SVGs until now. The new device assists physicians in meeting the standard of care in a broader range of SVG vessels.
The FilterWire EZ System is a low-profile embolic protection device that has been clinically proven to capture and remove embolic material, leading to reduced complications during balloon angioplasty and stenting procedures in SVGs. SVG disease occurs in patients who have previously had coronary artery bypass graft (CABG) surgery in which a vessel harvested from the patient’s leg is surgically attached to the arteries of the heart. Blood is redirected through the surgically attached SVG, bypassing the blocked artery and increasing blood flow to the heart.
The complex nature and progression of SVG disease as compared to native coronary artery disease can create a challenging treatment situation for physicians and a higher risk for patients. In Boston Scientific’s BLAZE II study, which evaluated the safety and performance of the FilterWire EZ System’s 2.25 - 3.5 mm size, a 30-day major adverse cardiac event (MACE) rate of 4.6 percent was reported, with MACE defined as death, myocardial infarction, emergent CABG or revascularization. The study also resulted in no deaths, no target lesion revascularizations (re-interventions) and no sub-acute thrombosis (clots) during the 30-day follow-up period. The BLAZE II study involved 131 patients in 16 sites in the United States.


 April 25, 2023
April 25, 2023