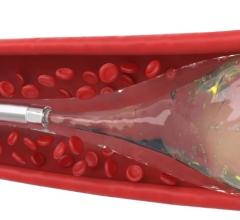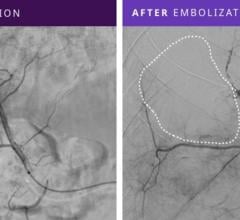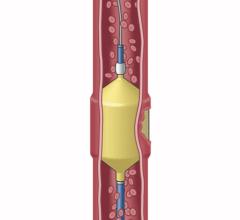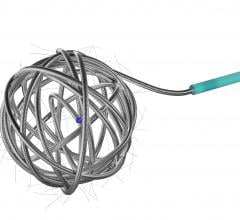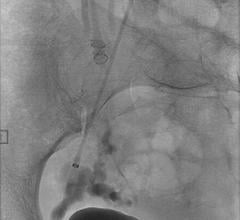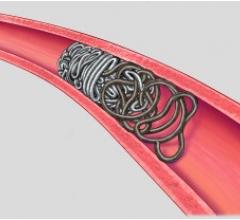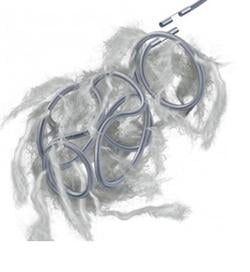
July 12, 2011 – The Interlock-35 Fibered IDC Occlusion System has been launched in the United States and Europe. Earlier this year, the system, manufactured by Boston Scientific, received clearance from the U.S. Food and Drug Administration (FDA) and CE mark approval for obstructing or reducing blood flow in the peripheral vasculature during embolization procedures. The Interlock-35 system consists of a 0.035-inch detachable coil that features a unique interlocking connection between the coil and delivery wire designed to offer excellent placement control, including the ability to advance, retract and reposition the coil before final deployment in the vessel. The coil is engineered to be accurately and reliably detached by simply pushing the detachment zone beyond the distal end of the 5 French delivery catheter. The platinum coil is constructed with a dense network of synthetic fibers, designed to offer excellent thrombogenicity (blockage of blood flow) and rapid stasis. "Compatibility with 5 French catheters allows for placement of larger coils, which can help achieve peripheral embolization with fewer coils, potentially reducing procedure times," said Sally Mitchell, M.D., professor of radiology, surgery and pediatrics at The Johns Hopkins University School of Medicine in Baltimore, Md. "The Interlock-35 coil provides excellent occlusive power while allowing precise retrievable placement, representing a major advantage over standard 0.035-inch pushable coil technology." The Interlock-35 Fibered IDC Occlusion System is available in 31 configurations that include coil lengths from 4 to 40 cm, diameters from 3 to 20 mm, and three distinct shapes (cube, 2-D helical and diamond) to offer physicians greater flexibility to treat diverse vessel anatomy. When combined with Boston Scientific's 0.018-inch Interlock Fibered IDC coils, the Interlock Coil portfolio provides 50 different coils to optimize peripheral embolization procedures. The company says the coil's user-friendly interlocking arms provide excellent control during deployment and detachment. The dense network of fibers provides rapid thrombosis, and the availability of larger and longer coils aid in effective vessel occlusion. For more information: www.bostonscientific.com


 June 05, 2025
June 05, 2025