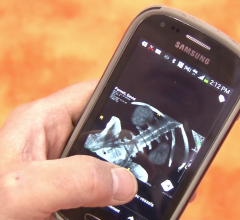
October 21, 2014 — Calgary Scientific Inc. has gained U.S. Food and Drug Administration (FDA) clearance of ResolutionMD 4.0 diagnostic medical imaging software for all modalities and China Food and Drug Administration (CFDA) certification for diagnosis on mobile devices in China.
This announcement follows the company’s recent news on gaining U.S. Food and Drug Administration (FDA) clearance for all modalities and China Food and Drug Administration (CFDA) certification for diagnosis on mobile devices in China.
The new version of ResolutionMD enables broader access to valuable information in a completely secure fashion by:
- Extending the view of a patient record across disparate systems and geographies by providing additional support for non-DICOM data types;
- Adding to the range of reporting systems supported, giving access to the complete patient record that is required to make informed clinical decisions; and
- Providing administrators with better control over how physicians access patient records in an enterprise, increasing security while allowing easy access to relevant information.
ResolutionMD enables doctors to securely view patient images and reports from a wide variety of computers and mobile devices, collaborate with other practitioners and diagnose from any location. The FDA cleared, CFDA certified, Health Canada licensed and CE marked mobile medical diagnosis software can be integrated into any electronic medical records (EMR) and easily plugs into existing distributed storage systems. Because of ResolutionMD’s federated approach, highly sensitive data is never moved to a device and no additional data storage locations are created.
For more information: http://offers.calgaryscientific.com/resolutionmd4

 April 05, 2019
April 05, 2019