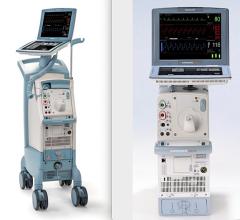
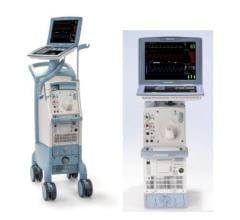
At TCT 2008, Datascope Corp. highlighted its Sensation 7 Fr. inter-aortic balloon catheter and the CS300 IABP with IntelliSense, which combine fiber-optic speed with automatic in vivo calibration for faster time to therapy, faster signal acquisition, and faster adaptation to rate and rhythm changes.
Datascope said it has the only fiber-optic IABP and catheter system that automatically calibrates in the patient after insertion and automatically recalibrates in vivo every two hours, or sooner should patient or environmental conditions change.
The New Sensation 7 Fr. is said to be the smallest IAB catheter available today.
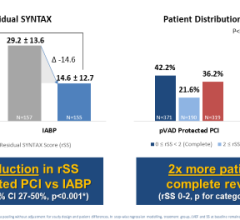
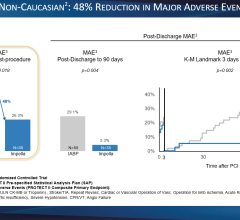
Datascope said IABP use can help significantly reduce infarct size, thereby improving myocardial salvage. Infarct size is a well-established surrogate clinical marker for patient mortality, congestive heart failure and quality of life.
IABC therapy is currently the gold standard for a variety of clinical indications. It is a Class I indication for cardiogenic shock in the ACC/AHA guidelines.
October 2008

 August 14, 2023
August 14, 2023