November 3, 2010 – The U.S. Food and Drug Administration (FDA) has approved a new stent delivery system for clinical practice in the United States. The Talent Thoracic Stent Graft with Captivia delivery system, by Medtronic, features a tip capture mechanism for controlled deployment and precise placement of the implantable medical device.
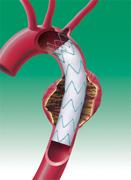
The system is used in the endovascular repair of thoracic aortic aneurysms (TAA). If left untreated, the dangerous bulges in the body’s main artery can be fatal.
During thoracic endovascular aortic repair (TEVAR), the system is inserted into the femoral artery and moved up through blood vessels to the aorta. When the device reaches the site of the aneurysm, the physician expands the stent graft within the aorta. This creates a new path for blood flow that reduces the risk of rupture.
"The Captivia Delivery System's tip capture mechanism is designed to provide excellent control of the stent graft during deployment to ensure that blood flow isn't occluded into the nearby arteries," said Edward Y. Woo, M.D., vice-chief and program director of vascular surgery and endovascular therapy for the University of Pennsylvania Hospital System. "This improvement to the delivery system also increases my confidence in the device's deployment accuracy."
The system features a hydrophilic coating to ease insertion into the femoral artery and navigation through the iliac arteries (around the pelvis) en route to the aorta.
For more information: www.medtronic.com

