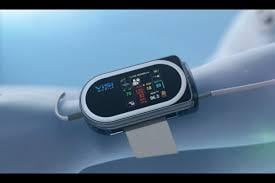
October 11, 2013 — The U.S. Food and Drug Administration (FDA) has cleared Sotera Wireless Inc.’s patented continuous non-invasive blood pressure (cNIBP) technology, a new feature of the ViSi Mobile wireless patient monitoring system. For the first time clinicians can continuously monitor all patient vital signs, including beat-to-beat blood pressure, without the use of a catheter or blood pressure cuff.
Physicians can use cNIBP measurements to better manage patients with hypertension, commonly known as high blood pressure. Hypertension affects 67 million, or one in three, American adults resulting in a direct healthcare cost of approximately $50 billion.
In the United States, hypertension is present in 69 percent of people who have a first heart attack, 77 percent of people who have a first stroke and 74 percent of people with chronic heart failure, placing it among the most dangerous risk factors for cardiovascular disease.
Numerous studies point to the benefits of beat-to-beat blood pressure monitoring, but obtaining accurate continuous data currently requires catheter insertion, exposing patients to discomfort and risk of infection. Sotera's cNIBP technology maximizes comfort and safety by obtaining measurements without invasive procedures or the frequent use of a blood pressure cuff. In studies comparing the clinically validated cNIBP technology with invasive arterial line procedures cNIBP technology demonstrated a bias of less than 5 mmHg.
"Blood pressure is one of the most important vital signs and yet it is considerably undervalued, largely because most clinicians are limited to periodic cuff-based measurements," said Tom Watlington, Sotera chief executive officer. "By providing data collected around the clock without disrupting a patient's sleep, we believe the ViSi Mobile System with cNIBP will help improve diagnosis and management of hypertension, saving lives and dollars."
The ViSi Mobile System is the first wrist-worn monitor to measure all core patient vital signs: cNIBP, heart rate or pulse rate, electrocardiogram (ECG), blood oxygenation level, respiration rate and skin temperature, with the accuracy of systems used in hospital intensive care units. Wireless technology transmits vital sign data, enabling clinicians to monitor measurements on remote viewing or notification devices. The system incorporates safety features, such as an advanced alarm management system that notifies clinicians of unfavorable changes in a patient's condition, enabling early intervention if needed.
For more information: www.soterawireless.com

 February 14, 2022
February 14, 2022