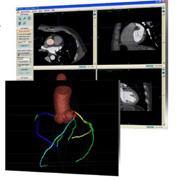
February 9, 2011 – The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for enhanced software that automatically detects significant (50 percent and more) stenotic lesions in coronary arteries from coronary computed tomography angiography (CTA) studies. The COR Analyzer system, from Rcadia Medical Imaging, is designed to help speed detection of coronary artery disease. "The enhanced version expands the benefits of the COR Analyzer System as a powerful complement to CTA in evaluating suspected CAD patients," said Shai Levanon, president and CEO of Rcadia. "By providing rapid detection of stenotic lesions our system enables expanded use of CTA to enhance patient care and reduce unnecessary costs." The FDA clearance follows the recent publication of the first clinical study evaluating the COR Analyzer's enhanced version. The study, published online in Academic Radiology in January 2011, demonstrated that the "COR Analyzer provides a high negative predictive value for the absence of coronary disease in branch vessels as well as the major coronary arteries." The retrospective study, conducted by Ethan Halpern, M.D., and David Halpern, compared 207 cases evaluated by the COR Analyzer System with expert interpretation. The final clinical interpretation identified 48 patients with significant (more than 50 percent) stenosis. The system demonstrated a sensitivity of 92 percent and a negative predictive value of 97 percent. In the study, the system's specificity was 70 percent and the positive predictive value was 48 percent. The investigators noted that the high negative predictive value will be most useful in a population with a low prevalence of coronary disease, such as the emergency room chest pain population. In these populations, the system could be used to facilitate the rapid discharge of a majority of patients who have normal coronary CTA studies. For more information: www.rcadia.com

 May 12, 2020
May 12, 2020