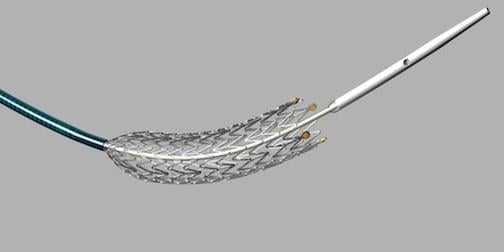
November 15, 2012 — The U.S. Food and Drug Administration (FDA) granted market clearance for Cook Medical’s Zilver PTX drug-eluting peripheral stent. It is the first device of its kind on several fronts to go before FDA review. It is the first drug-eluting stent (DES) for peripheral leg vessels, the first self-expanding DES and the first DES that does not use a polymer to load and elute its drug.
The Zilver PTX Drug-Eluting Stent is intended to treat peripheral arterial disease (PAD) in the superficial femoral artery (SFA). The stent is seen by many experts as a major step forward in treatment options for patients with peripheral artery disease (PAD) in the SFA. Occlusions in the SFA are difficult to treat and frequently re-occlude.
“With this approval, treating PAD in the U.S. will begin to undergo the same revolution that drug elution did for treating coronary artery disease,” added Gary Ansel, M.D., director for the Center for Critical Limb Care at Riverside Methodist Hospital in Columbus, Ohio, and an assistant clinical professor of medicine in the Department of Internal Medicine at the University of Toledo Medical Center in Toledo, Ohio. “Drug-eluting stents such as Zilver PTX will move quickly, in my opinion, to become the standard of care for PAD patients worldwide.”
Zilver PTX is made of nitinol, a shape memory metal alloy that will spring back into its original shape under axial, torsional and bending flexibility. Self-expanding stents are used in the SFA because of the need to torque and compress with the vessel.
The addition of the anti-tissue proliferation drug paclitaxel on the Zilver stent has shown promising results in clinical trials in lowering the usually high restenosis rates of SFA lesions.
Polymers used to load drugs and control drug elution on DES have been implicated as the culprit in late-stent thrombosis sometimes seen in coronary stents. Vendors have been researching ways to eliminate polymers, allowing DES to become a bare metal stent following full drug elution into the vessel wall. The Zilver PTX would be the first DES in the United States not to use polymer, which is seen by many interventional experts as the way of the future.
In order to supply as many physicians as possible with this new technology, Cook is making Zilver PTX available initially in 80 mm lengths in 6 mm and 7 mm diameters. The product's indications for use also allow two Zilver PTX 80 mm stents to be overlapped to treat longer lesions up to 140 mm. The FDA approval also includes 40 mm and 60 mm lengths, which will be introduced to the U.S. early in 2013. Cook expects to receive regulatory approval for 120 mm length stents in both diameters next year.
Data from Cook’s pivotal clinical trial indicate:
· Eight out of ten patients treated with Zilver PTX still had open arteries (primary patency) after one year. That compares to only three out of 10 patients treated with angioplasty alone.
· Patients who received a bare metal stent required more than twice as many reintervention procedures to reopen the SFA as patients who received Zilver PTX.
“After conducting the largest randomized controlled study of peripheral stenting ever undertaken, we now see remarkable results in patients treated with Zilver PTX,” said Michael Dake, M.D., a professor in the Department of Cardiothoracic Surgery at Stanford University School of Medicine and medical director of the cath/angio laboratories at Stanford Medical Center, Palo Alto, Calif.
The stent gained European CE mark approval in 2009. The device is being introduced to the U.S. market in a five-step process designed to make this technology available to as many patients as possible initially.
For more information: www.cookmedical.com


 November 14, 2025
November 14, 2025 









