Siemens Healthcare announced that syngo Neuro PBV IR (parenchymal blood volume interventional radiology) has received FDAmarketing clearance. The software package is intended to provide visual assistance to physicians in the diagnosis and treatment of vessel malformations.
July 25, 2011 —Siemens Healthcare announced that syngo Neuro PBV IR (parenchymal blood volume interventional radiology) has received U.S. Food and Drug Administration (FDA) 510(k) marketing clearance. The software package is intended to provide visual assistance to physicians in the diagnosis and treatment of vessel malformations (such as aneurysms, arteriovenous malformations and stenoses).
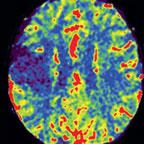
In neuroradiology, this feature assists physicians in the treatment of stroke patients by displaying a color-coded qualitative map of cerebral tissue directly in the angiography suite. syngo Neuro PBV IR further expands the Siemens imaging application portfolio for the Artis zee line of interventional X-ray systems for radiology and cardiology.
Strokes result from decreased blood flow in the brain and can cause irreparable damage to cerebral tissue. Early treatment of a stroke can help minimize damage to brain tissue. Treatment generally involves minimally invasive techniques, such as image-guided, catheter-based interventions where blood clots are either dissolved with medication or mechanically removed. syngo Neuro PBV uses cone-beam computed tomography (CT) technology to produce a qualitative colorized image similar to CT perfusion. It is designed for the visualization of contrast-enhanced blood distribution in the arterial and venous vessels in the head using color-coded relative values for diagnosis.
“Neurovascular imaging has evolved from simple anatomical 2-D rendition of blood vessels to 3-D spatial visualization and, with the Neuro PBV, to physiological functional imaging of the brain,” said Michel E. Mawad, M.D., Baylor College of Medicine, Houston. “Neuro PBV is a promising technique that will add valuable information about the viability of the brain and should help guide the interventionalist in the decision-making process of revascularization procedures."
Parenchymal blood volume information is acquired with two C-arm rotations around the patient and a steady-state contrast injection. The system applies sophisticated processing algorithms to generate a neurological PBV map. The information is available at tableside in less than 40 seconds without the need for any further user interaction.
For more information: www.siemens.com/healthcare


 October 24, 2025
October 24, 2025 









