June 12, 2014 — The U.S. Food and Drug Administration (FDA) today expanded the indicated use for the CoreValve self-expanding transcatheter aortic valve system for patients with severe aortic stenosis who are at high risk for surgery. CoreValve originally gained FDA clearance in January for patients at extreme risk for surgical valve replacement. This new approval is based on groundbreaking data that showed clinical outcomes at one year with the CoreValve system were superior to the current gold standard of open-heart surgery.
The FDA approved the CoreValve for high-risk patients without the need for an independent device advisory panel review due to its exceptionally positive clinical results demonstrated in the high-risk study of the CoreValve U.S. pivotal trial. The head-to-head study, comparing transcatheter aortic valve replacement (TAVR) with the CoreValve system to traditional surgical aortic valve replacement, met its primary endpoint with high survival at one year for patients receiving the CoreValve (85.8 percent), which was statistically superior to patients receiving a surgical valve (80.9 percent).
“This rigorous trial has defined a new standard for transcatheter valve performance, with superiority results that give physicians even more confidence in making TAVR treatment decisions,” said David H. Adams, M.D., chair of the department of cardiothoracic surgery at the Mount Sinai Hospital, New York City, and national co-principal investigator of the CoreValve U.S. pivotal trial. “With this approval, we can treat more patients due to the broad range of CoreValve sizes, and we have an option compared to surgery that provides a greater chance for a longer life while minimizing the risk of stroke.”
View video interview with David Adams on the CoreValve trial at ACC.14.
As a principal investigator in the Medtronic CoreValve U.S. pivotal trial, Robert C. Stoler, M.D., FACC, FSCAI, led the study of patients at Baylor Heart and Vascular Hospital (BHVH). The hospital was one of 45 national trial sites and the only site in northern Texas to participate in the research.
“This is the first time we’ve seen a transcatheter-based therapy do better than open-heart surgery patients,” said Stoler. “That really opens up a door for treating patients in the future with a far less invasive method of valve replacement.”
According to the trial findings, released at the American College of Cardiology’s (ACC) 2014 scientific sessions and published in the New England Journal of Medicine, patients with the CoreValve TAVR device experienced significantly improved survival ratings at one year and showed better quality of life indicators at 30 days, compared with open-heart participants.
“And those results are just the tip of the iceberg,” said Stoler, adding that the Baylor Research Institute (BRI) is involved in several other studies exploring TAVR’s application to valve patients. “There are new generations of valves coming out from several different manufacturers, and we’re interested to see how those affect outcomes, including stroke.”
"If you’re a patient and you need an aortic valve replacement, you have more options now,” he said. “Transcatheter valve therapy is going to expand to a bigger patient population, and we’re seeing more patients who now have a chance to be treated with valve replacement. Plus, the new generations of valves are going to have smaller catheter sizes and fewer complications. They’re only getting better.”
For patients treated with the CoreValve system in the high-risk study, rates of stroke — one of the most concerning complications of valve replacement because it increases the risk of death and can have a dramatic impact on quality of life — were low and not statistically different than rates experienced by surgery patients. The rate of MACCE (major adverse cardiovascular or cerebral events) was significantly better for CoreValve patients at one year, and overall hemodynamic performance was better in CoreValve patients than in surgical patients across all time points.
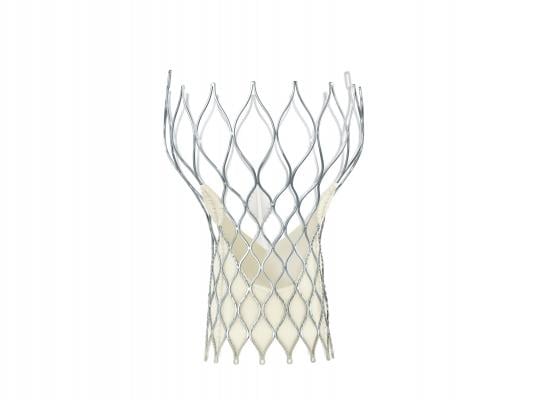
CoreValve Device Details
The CoreValve system was designed to serve the clinical needs of the broadest range of patients with aortic stenosis. The valve’s self-expanding frame provides controlled deployment, enabling physicians to accurately place the valve inside a patient’s original valve, while conforming to the native annulus to provide a seal. The FDA approved the entire CoreValve platform, including the 23, 26, 29 and 31 mm size valves. All of these sizes are delivered through an 18 French delivery system, which is the smallest commercially available TAVR delivery system.
Medtronic worked closely with the FDA throughout the pivotal clinical trial and premarket approval (PMA) review process. FDA granted priority review designation for both the extreme-risk and high-risk PMA submissions. Priority designation is granted to new therapies of major public health interest. Based on the strength of the trial data, FDA determined that sufficient information was available to evaluate the safety and efficacy of the Medtronic CoreValve system for both patient groups without the need for external advisory committee panels.
Since receiving CE mark in 2007, the CoreValve system has been implanted in more than 60,000 patients in more than 60 countries.
The CoreValve is the second TAVR system to gain FDA clearance. The first was the balloon-expandable Edwards Lifesciences Sapien valve.
For more information: www.corevalve.com

