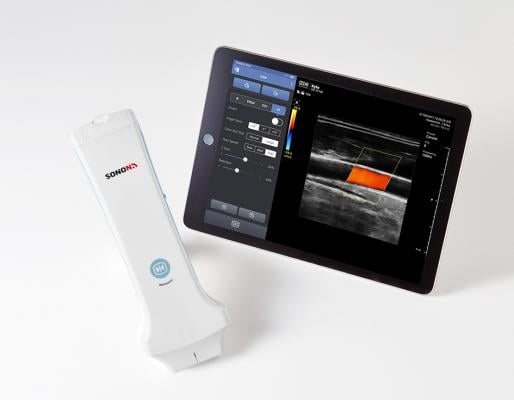
July 9, 2018 — South Korea-based Healcerion launched the Sonon 300L wireless handheld ultrasound device to the U.S. market following U.S. Food and Drug Administration (FDA) approval. The Sonon 300L is designed to help primary care providers (PCPs) be more efficient and effective, with instant insight to diagnose or refer a patient to a specialist. The system provides flexible ultrasound technology at less than 1/10 the cost of a traditional ultrasound machine, with a user interface anyone can learn in minutes, according to the company.
Sonon 300L is an advanced diagnostic imaging device that can be used anywhere, with a mobile app downloaded from the Google Play or Apple App Store.
The system also supports medical imaging protocols including DICOM (Digital Imaging and Communications in Medicine) and PACS (picture archiving and communication systems). Using intuitive finger-touch control to operate, it provides rapid, accurate diagnostics to improve patient care and improve efficiency. Sonon 300L has a three-hour continuous scanning battery life, which far exceeds other handheld ultrasound devices, according to Healcerion.
Currently, according to the February 2018 Journal of Family Practice, less than 10 percent of PCPs in the United States utilize ultrasound technology for diagnostic use - for example to assess thyroid, carotid, breast, lung and vascular conditions. In other countries, a larger percentage of physicians utilize diagnostic ultrasound in primary care.
Sonon 300L also provides a new diagnostic ultrasound option for retail clinics, urgent care centers, mini-hospitals, home healthcare providers, and rural and third-world regions where cost, space and mobility requirements had previously put traditional ultrasound out of reach.
For more information: www.healcerion.com

 April 05, 2019
April 05, 2019