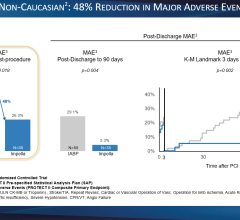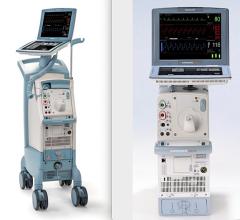
September 20, 2011 — Maquet Cardiovascular announced it has received both 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Sensation Plus 50 cc 8 French intra-aortic balloon (IAB) catheter. The device also received CE mark approval from the British Standards Institution (BSI).
The new catheter incorporates fiber optic signal acquisition and provides 25 percent more blood volume displacement than standard 40cc IAB catheters, allowing for improved unloading and better augmentation. It also comes with two Stat Lock IAB stabilization devices that allow the catheter to be secured to the patient's leg without sutures. This is more comfortable for patients and eliminates the risk of suture needle sticks for clinicians when initiating counterpulsation support.
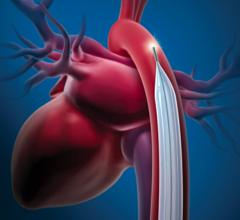
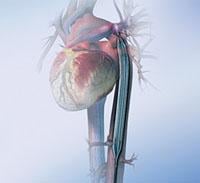
Intra-aortic balloon counterpulsation is an adjunctive therapy often used in patients with left ventricular failure and other cardiac conditions. When the IAB, inserted into the patient's aorta, counterpulsates with the heart, it augments coronary blood flow to increase myocardial oxygen supply and decrease myocardial oxygen demand.
Sensation Plus will be available for sale in October 2011.
For more information: http://ca.maquet.com

 August 14, 2023
August 14, 2023