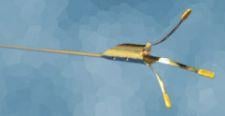
The Ostial Pro Stent Positioning System is designed to facilitate precise stent implantation in coronary and renal aorto-ostial lesions, eliminating the guesswork when deploying a stent at the “true” ostium of the vessel.
The gold-plated feet of the Ostial Pro mark the plane of the aortic wall, allowing precise positioning of the stent relative to the true ostium. The device is compatible with any manufacturer’s 6 Fr, 7 Fr or 8 Fr guiding catheter and any manufacturer’s stent platform.
Ostial Solutions LLC said clinical testing demonstrates stent positioning accuracy to within /- 0.5 mm.
The Ostial Pro helps minimizes the risk of the stent being deployed too proximal to the ostium, the maker said. Should the stent protrude into the aorta, future vessel re-engagement becomes very challenging, often leaving CABG as the patient’s only available revascularization option.
The system is also said to reduces the need for a second stent, because if the stent is deployed distal to the ostium, an additional stent is typically required. The company said a second stent increases costs and can cause metal overlap, thrombosis risk and associated restenosis.
The device can help decreases overall procedure time by reducing fluoro time and contrast use typically required to place an aorto-ostial stent, the company said.


 November 24, 2025
November 24, 2025 









