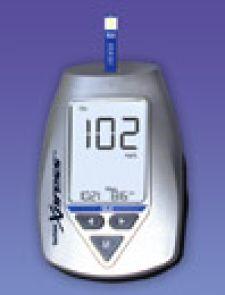
A new, low-cost hospital blood glucose meter from Nova Biomedical provides the same analytical performance of Nova's patented Multi-Well test strip for those testing situations where glucose meter interface capability is not needed.
The introduction of StatStrip Xpress provides hospitals a choice of glucose monitors with or without a connectivity solution using the same Multi-Well test strip.
Like the StatStrip Glucose Hospital Meter, StatStrip Xpress offers central lab quality bedside glucose testing, the company said. It incorporates a new strip technology that uses four measuring wells. Nova�s Multi-Well system measures and corrects hematocrit interference, as well as interferences from acetaminophen (Tylenol), uric acid, ascorbic acid (vitamin C). StatStrip Xpress also eliminates oxygen, maltose, galactose, and xylose interferences.
StatStrip Xpress has a six-second analysis time using a 1.2 microliter sample size. Unlike competitive glucose analyzers, StatStrip Xpress requires no calibration codes. Nova said this eliminates a potential error source, reduces the number of steps required for testing, and allows StatStrip Xpress manufacturing lots to be used interchangeably.
March 2008


 August 05, 2019
August 05, 2019 








