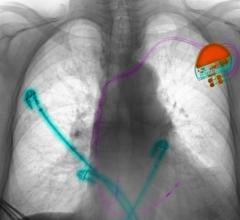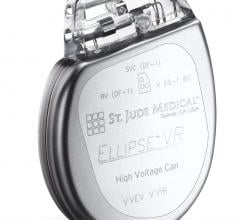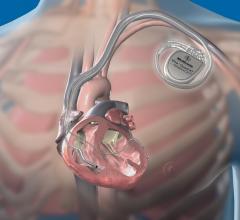
June 10, 2009 - St. Jude Medical Inc. today announced the first implant of its Current Plus implantable cardioverter defibrillator (ICD), featuring the SJ4 connector system.
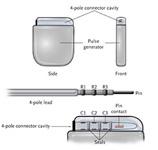
The SJ4 connector reduces the number of connections between the device and the wires (defibrillation leads) that send electrical impulses to the heart, which is intended to enable a streamlined implant procedure.
The SJ4 connector system features a single connection between the device and the defibrillation lead, and a single set screw (used to tighten and secure the lead to the device). Previous defibrillator lead designs required three separate connections and four set screws. The reduced number of lead connections also lessens the risk of lead-to-can abrasion, a known complication that can occur in patients who have an implantable device.
“With only a single connection and one set screw, the SJ4 connector has the potential to improve the implant procedure, may reduce the lead volume under the ICD in the chest wall and may improve patient comfort,” said Cleveland Clinic’s Bruce Wilkoff, M.D., who is on the company’s physician lead review board and has sponsored research with St. Jude Medical Inc. Dr. Wilkoff implanted the first Current Plus ICD with SJ4 connector on June 4. “This design is intended to reduce the risk of incorrect connections of the lead to the ICD and reduce procedure time.”
The St. Jude Medical SJ4 connector system is designed to meet the draft IS-4 standard as set forth by the International Organization of Standardization (ISO), but will not be labeled as such until the standard is finalized, which is expected later this year. St. Jude Medical began launch of the SJ4 connector system after ISO-directed interchangeability testing among multiple manufacturers was completed. This testing was deemed an important step in ensuring that these new leads, which currently meet the drafted IS-4 standard, would be compatible with future implanted devices.
The Current Plus ICD was approved by the FDA in April 2009, along with the company’s Promote Plus cardiac resynchronization therapy defibrillator (CRT-D), which are compatible with the Durata SJ4 defibrillation lead. As with previously announced leads in the Durata lead family, the Durata lead with SJ4 connector features a soft silicone tip and Optim insulation, a hybrid insulation material that provides increased abrasion-resistance and durability, along with the flexibility and handling characteristics that facilitate device implantation.
Both devices are built on the St. Jude Medical Unity platform, a consolidated hardware and software unified device interface, and include advanced safety features and algorithms for improved patient management, including TailoredTherapy features that allow physicians to customize therapy to individual patient needs. The devices also feature improved lead monitoring capabilities, including daily checks of all pacing and shock configurations, and have the ability to inform the patient’s clinic, via the St. Jude Medical Merlin@home transmitter and Merlin.net Patient Care Network (PCN), of any critical system changes.
For more information: www.sjm.com

 February 24, 2023
February 24, 2023