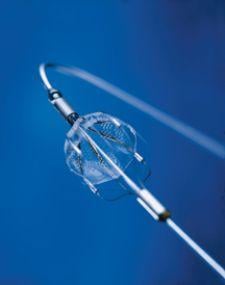
Cordis Corp. has received approval from the FDA to market its PRECISE Nitinol Stent and ANGIOGUARD Emboli Capture guide wire. The system is used to treat carotid artery disease in patients at high risk for adverse events from carotid endarterectomy (CEA) — a surgical procedure for removing arterial plaque from the carotid artery.
The PRECISE with ANGIOGUARD is the only carotid system backed by a large, randomized clinical trial — the SAPPHIRE study — to support the potential benefits of carotid artery stenting (CAS) in patients who are ineligible, or considered high-risk, for carotid endarterectomy. The carotid system has been studied in over 3,000 patients across both SAPPHIRE and the Carotid Artery Stent Education System Post-Marketing Study and demonstrated low stroke rates in both.


 November 24, 2025
November 24, 2025 









