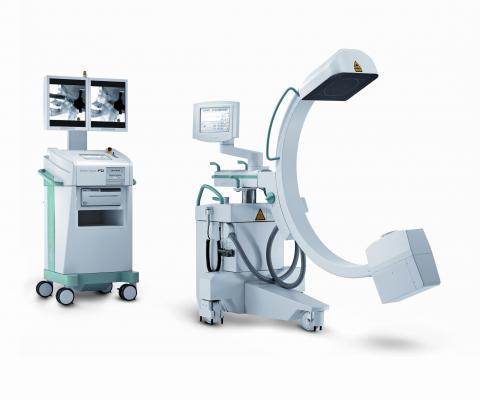
Image courtesy of Ziehm Imaging Inc.
December 15, 2015 — Toshiba America Medical Systems Inc. is now making mobile C-arms available to its customers in the United States through a new partnership with Ziehm Imaging Inc. This strategic relationship offers customers a broader range of vascular and surgical imaging technologies with access to mobile C-arms from Ziehm, while Ziehm customers benefit from fixed C-arm systems of Toshiba’s Infinix product line.
Mobile C-arm technology allows for portable and cost-effective solutions for interventional and surgical imaging. Ziehm Imaging offers flexibility, efficiency and advanced safety features to help Toshiba customers provide the right imaging and treatment for their patient’s needs. Additionally, Toshiba’s Infinix line will give Ziehm Imaging Inc. customers the image quality, flexible system mechanics and patient safety features that are necessary for more complex cases. Product offerings in cardiac, neuro and even combined angiographic and computed tomography (CT) imaging will give Ziehm Imaging Inc.’s customers the right capabilities for a variety of situations.
For more information: www.medical.toshiba.com, www.ziehm.com


 October 24, 2025
October 24, 2025 









