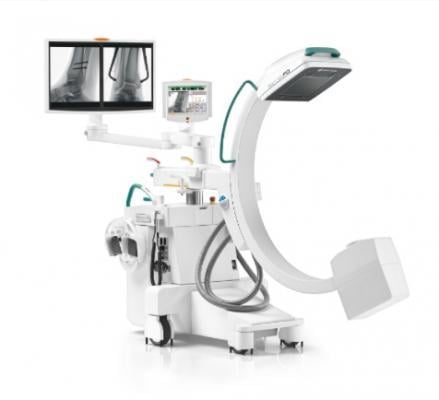
November 27, 2016 — At the 2016 Radiological Society of North America (RSNA) annual meeting, Ziehm Imaging presents its latest generation of C-arms, including the Ziehm Solo FD and Ziehm Vision RFD. Both systems feature CMOS flat-panel (FD) and according to the company are the first full-size C-arms equipped with this new technology. Further highlights are the new Ziehm NaviPort 3-D interface for image-guided navigation to improve reliability and precision during surgical procedures, and the Ziehm Vision RFD Hybrid Edition, a comprehensive mobile hybrid solution for cardiovascular procedures.
The new flat-panels feature CMOS technology as an alternative to amorphous silicon detectors and enable higher image resolution at the same dose, closing the gap between the image quality of flat-panel technologies and the cost efficiency of image intensifiers. The new CMOS detectors will be on display on the Ziehm Vision RFD and the Ziehm Solo FD. The Ziehm Solo FD is already commercially available. Due to its versatile design, the Ziehm Solo FD ensures maximum flexibility. The mobile C-arm provides optimal soft tissue and bone contrast especially in orthopedic, trauma and pain management procedures.
The Ziehm Vision RFD comes with a 25 kW power generator for demanding vascular procedures and will become commercially available with CMOS technology in early 2017.
OrthoScan, the specialist for mini C-arms, will present its portfolio of mini C-arms at RSNA and will showcase a Ziehm Solo FD in their booth as well.
The new Ziehm NaviPort interface for image-guided navigation connects the Ziehm Vision RFD 3-D seamlessly with systems of leading navigation companies. The combination of intraoperative 3-D imaging and navigation systems enables clinicians to ideally manage less-invasive approaches for shorter hospital stays and improved patient outcomes.
The Ziehm Vision RFD Hybrid Edition is, according to the company, the first fully motorized mobile C-arm. The system is a space and cost saving alternative to fixed installed systems, as it does not require any room preparation. Due to its low installation and operating costs, the Ziehm Vision RFD Hybrid Edition is a comprehensive mobile hybrid solution for highly demanding cardiovascular procedures.
For more information: www.ziehm.com


 October 24, 2025
October 24, 2025 









