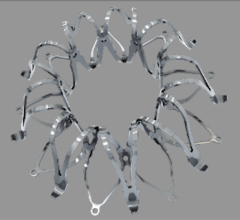
June 24, 2014 — OrbusNeich announced the completion of enrollment in the prospective, multicenter, all-comers REMEDEE Registry (Multicenter, Prospective, Clinical Outcomes After Deployment of the Abluminal Sirolimus Coated Bio-Engineered Stent (Combo Bio-Engineered Sirolimus Eluting Stent) Post Market Registry) to evaluate the Combo stent for the treatment of coronary lesions in the setting of routine clinical care. The registry enrolled 1,000 patients at nine European high-volume percutaneous coronary intervention centers in France, Latvia, Luxembourg, The Netherlands, United Kingdom and Spain.
The registry's primary endpoint is clinically driven target vessel failure (TVF) at one-year post procedure, which includes cardiac death, target vessel myocardial infarction (MI) and ischemia driven target vessel revascularization (TVR).
"The REMEDEE Registry will evaluate the long-term safety and performance for the COMBO Stent in a real-world patient population," said Robbert de Winter, M.D., Ph.D., of the Academic Medical Center, Amsterdam, and principal investigator of the study. "This innovative device allows for accelerated stent endothelialization and healing and therefore addresses the challenge of delayed arterial healing caused by monotherapy drug eluting stents. We expect primary endpoint data in the second quarter of 2015."
Secondary endpoints include device and procedural success and adjudicated target lesion failure (TLF) at 30 and 180 days post procedure. Additional secondary endpoints to be evaluated at 30, 180 and 365 days include the components of TVF, defined as cardiac death, target vessel MI and ischemia driven TVR; adjudicated major adverse cardiac events (MACE) as a composite and as each of its components, defined as death, any MI and any revascularization; and adjudicated stent thrombosis. Clinical follow-up will be conducted each year up to five years.
For more information: www.OrbusNeich.com

 May 02, 2024
May 02, 2024