Sept. 23, 2025 — CeleCor Therapeutics’ multinational Phase 3 clinical trial of its investigational heart-attack drug ...
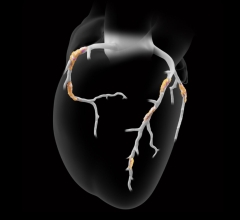
Sept. 22, 2025 — Heartflow, Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Next Gen ...
Sept. 22, 2025 — The latest findings on heart failure (HF) published by the Heart Failure Society of America (HFSA) ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Sept. 18, 2025 — The Society of Nuclear Medicine and Molecular Imaging (SNMMI), American Society of Nuclear Cardiology ...
Sept. 18, 2025 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for treating cardiovascular and ...
Sept. 16, 2025 — Elutia Inc., a pioneer in drug-eluting biomatrix technologies, has published clinical and preclinical ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Sept. 16, 2025 — Conavi Medical Corp., a commercial-stage medical device company focused on designing, manufacturing and ...
Sept. 17, 2025 — Imperative Care recently announced positive efficacy and safety results from the pivotal Symphony-PE ...
Sept. 8, 2025 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Microbot Medical's Liberty ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Sept. 17, 2025 — Barbara Riegel, Ph.D., R.N., FAHA, Emerita Edith Clemmer Steinbright Professor of Gerontology at the ...
Sept. 16, 2025 — PaceMate has appointed Sean Shoffstall as Head of AI, Innovation and Data Strategy. Shoffstall joins ...
Sept. 11, 2025 — Newswise — A new Mayo Clinic study finds that many heart attacks in people under 65 — especially women ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Sept. 9, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on safely and effectively treating ...
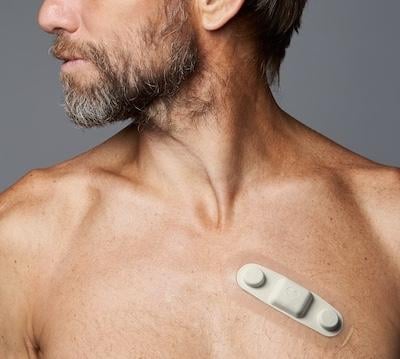
Atrial fibrillation (AFib) is one of the most common heart rhythm disorders, but researchers still don’t have a reliable ...
Sept. 2, 2025 — Although cardiovascular disease is a leading cause of death for women, they remain underrepresented in ...


 September 23, 2025
September 23, 2025