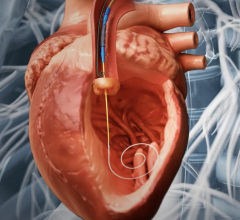
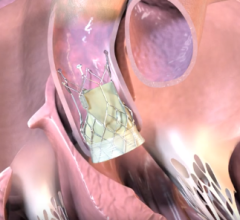
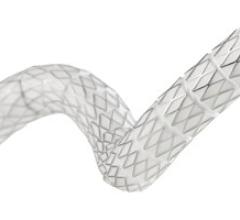
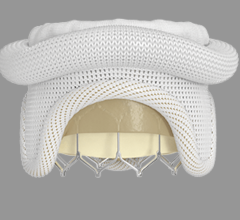
October 26, 2022 — Thousands of people have new hope for treatment of thoracic aortic arch disease and University Hospitals (UH) Harrington Heart & Vascular Institute is at the forefront of studying ...
Endovascular Aortic Repair
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
January 31, 2024 — As part of efforts to continuously improve medical solutions for patients with complex vascular ...
December 26, 2023 — MindMics, Inc. reported results from a clinical study of revolutionary earbuds that use a new ...
December 20, 2023 — The Smidt Heart Institute at Cedars-Sinai has opened an Aortic Surveillance Clinic for the ...
December 12, 2023 — Patients who received the anticoagulant drug warfarin after bioprosthetic aortic valve replacement h ...
October 30, 2023 — -egnite, Inc., a leading cardiovascular digital health company, announced today that novel research ...
October 19, 2023 — OpSens, a medical device cardiology-focused company, has announced that results of the “SAFE-TAVI ...
July 21, 2023 — Onecrea Medical has announced the success of the first-in-human implants of its Transcatheter Aortic ...
May 15, 2023 — The Cardiovascular Research Foundation (CRF) announced that TVT: The Structural Heart Summit will feature ...
January 12, 2023 — Medtronic announced the first patient enrollment in the ADVANCE Trial, a head-to-head randomized ...
December 30, 2022 — Azmi Atiya, MD, an established and highly respected cardiothoracic surgeon in the San Fernando ...
December 14, 2022 — Edwards Lifesciences identified the top data releases from 2022 that contributed most to shaping ...
December 5, 2022 — Imagen Technologies, Inc. announced the U.S. Food and Drug Administration's 510(k) clearance of the ...
November 2, 2022 — The second round of late-breaking clinical trial results were announced at VIVA22 on Nov. 1 in Las ...






 January 31, 2024
January 31, 2024