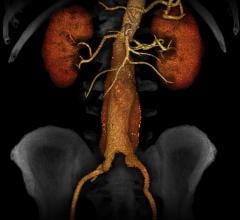
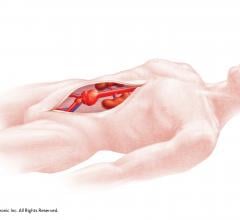
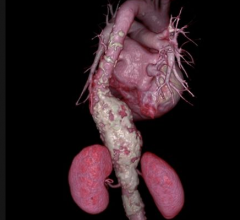
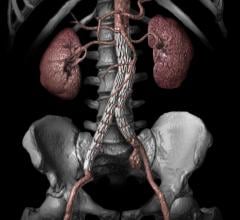
September 18, 2015 —Cook Medical has received premarket approval from the U.S. Food and Drug Administration (FDA) for ...
Endovascular Aortic Repair
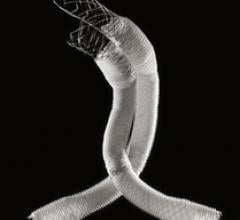
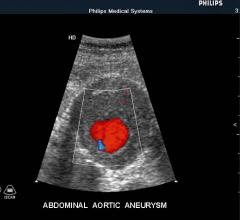
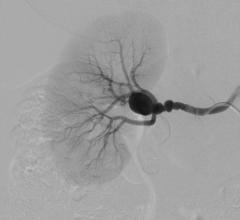
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
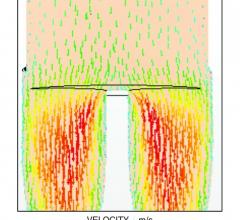
August 27, 2015 — The use of computational fluid dynamics (CFD) modeling has been used for years to better engineer ...
August 19, 2015 — Lombard Medical Inc. a medical device company focused on endovascular aneurysm repair (EVAR) of ...
August 13, 2015 — Medtronic plc announced that the first two European patient cases with the Endurant Evo AAA stent ...
July 2, 2015 - Medtronic plc announced it has acquired the assets of Aptus Endosystems Inc., a privately held medical ...
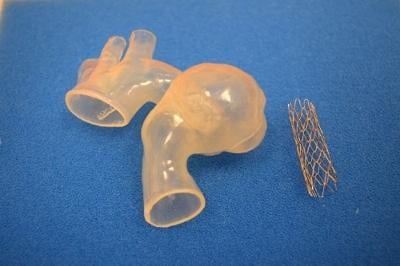
April 2, 2015 — Seventeen-year-old Ariana Smith recently became the first patient at the Children's Hospital of Michigan ...
March 12, 2015 — St.Vincent Heart Center is first in Indiana and second in the nation to participate in a clinical study ...
February 5, 2015 — A new computer program allows doctors to assess blood flow as they are using flow-diverter devices to ...
January 9, 2015 — W. L. Gore & Associates Inc. announced that the Gore Viabahn Endoprosthesis has received CE Mark ...
December 23, 2014 — Vital Images Inc. received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
December 10, 2014 — Medtronic Inc. announced the launch in Europe and the United States of the Endurant IIs AAA stent ...
November 20, 2014 — Oxford University researchers presented data that reveal the extent to which smoking causes silent ...
November 18, 2014 — Pierre Galvagni Silveira, M.D., Ph.D., from the Federal University of Santa Catarina in ...
November 17, 2014 — VIVA Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
October 22, 2014 — Lombard Medical Inc., a medical device company focused on endovascular aneurysm repair (EVAR) of ...

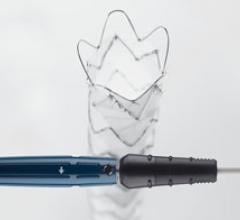
 September 18, 2015
September 18, 2015