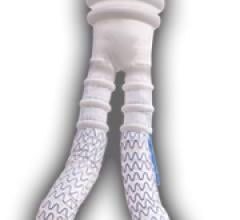
December 22, 2011 — TriVascular Inc. announced the first Canadian patients treated with its Ovation Abdominal Stent ...
Endovascular Aortic Repair
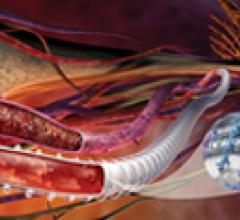
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
December 14, 2011 — Aptus Endosystems Inc. announced it received 510(k) clearance from the U.S. Food and Drug ...
December 6, 2011 — W. L. Gore & Associates, Inc. (Gore) has received U.S. Food and Drug Administration (FDA) approval ...
November 23, 2011 – Vascular occlusions are blocked blood vessels caused by a blood clot in an artery. Blocks in the ...
November 23 2011 – At the 2011 VEITH symposium, Arno von Ristow, M.D., department of vascular and endovascular surgery ...
November 23, 2011 – The U.S. Food and Drug Administration (FDA) allowed marketing of the first system that can repair a ...
November 22, 2011 — The thoracic aorta can pose major problems for endovascular repair when there are tortuous segments ...
November 22, 2011 — The question remains whether patency rates for heparin-bonded polytetrafluoroethylene (PTFE) grafts ...
November 18, 2011 – W. L. Gore & Associates Inc. announced that it has received approval from the U.S. Food and Drug ...
November 4, 2011 — The U.S. Food and Drug Administration (FDA) has approved a stent graft system for patients with small ...
The space needed and costs involved to create a hybrid operating room (OR) for a specific speciality may not be feasible ...
September 12, 2011 — TeraRecon Inc. invited interventional specialists to “Intervene with iNtuition,” the company’s ...
August 31, 2011 — Medtronic Inc. announced the start of its United States post-approval study of the Endurant AAA stent ...
August 19, 2011 - Endologix Inc. said a ruling this week will likely aid its defense in a stent graft patent litigation ...
August 12, 2011 — Endologix Inc. announced that the first American commercial implant of the company's AFX Endovascular ...


 December 22, 2011
December 22, 2011