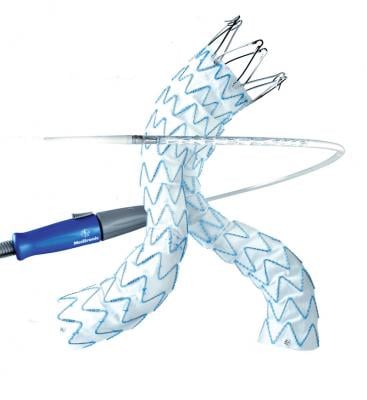
July 15 2014 — Researchers at the University of California, San Diego School of Medicine have documented the safety benefits of aortic stent grafts inserted during minimally invasive surgery to repair abdominal aortic aneurysms in a study published July 9 in JAMA Surgery.
The study shows that patients who received the minimally invasive aortic repair procedure had a 42 percent reduction in preventable post-operative complications and a 72 percent reduction in mortality, compared with those who had undergone open repair surgery.
The safety of the endovascular "inside blood vessel" procedure also appears to be improving over time, as researchers documented a 37 percent reduction in the likelihood of an avoidable complication between 2003 and 2010.
The statistics are based on an analysis of 70,946 cases of abdominal aortic aneurysm repair performed over the seven-year period, culled from a nationwide hospital database maintained by the Healthcare Cost and Utilization Project.
"All this is good news for patients because endovascular repair has become the most common treatment for abdominal aortic aneurysms," said senior author John Lane, M.D., director of endovascular surgery at UC San Diego Health System and associate professor at the UC San Diego School of Medicine. The lead author is John Rose, M.D., a resident physician at UC San Diego School of Medicine.
Endovascular aortic aneurysm repair involves accessing the damaged aorta by first puncturing a blood vessel in the groin, often without making an incision, and then inserting a metallic stent that is guided using X-ray to the target area where it is then expanded. By contrast, in open repair surgery, surgeons make a large incision in the abdomen and manually sew a reinforcement graft into place.
"We have known that minimally invasive procedures are safer for patients," said Lane, who is also chief of endovascular surgery at Veterans Affairs San Diego Healthcare System. "It has been shown in randomized clinical trials and noted anecdotally. This is, however, the first time that we have been able to show that endovascular aneurysm repair is safer in terms of preventing complications in the hospital, as measured by patient safety indicators."
The value of the study, he said, is that it rigorously documents the benefits within the context of more than a dozen formally tracked patient safety indicators (PSIs), included in nationwide hospital care databases to help monitor and prevent avoidable complications during hospitalizations.
These PSIs include wound infection, blood infection (sepsis), hip fracture, accidental puncture or laceration and transfusion reaction and mortality among patients diagnosed as low-risk.
The findings represent a repeatable, statically defensible method for evaluating the comparative safeties of different surgical treatment options, particularly new methods.
"Medical errors and patient safety are an ongoing concern with any new surgical innovation," said David C. Chang, Ph.D., MPH, MBA, director of outcomes research at UC San Diego School of Medicine and a co-author. "This study shows the value in monitoring the safety of innovations. Patients need to keep this type of information in mind when considering different treatment options."
For more information: www.health.ucsd.edu


 January 05, 2026
January 05, 2026 









