October 26, 2022 — Thousands of people have new hope for treatment of thoracic aortic arch disease and University Hospitals (UH) Harrington Heart & Vascular Institute is at the forefront of studying ...
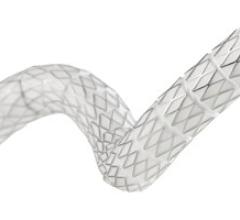
Stent Grafts
Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
January 31, 2024 — As part of efforts to continuously improve medical solutions for patients with complex vascular ...
January 12, 2023 — Medtronic announced the first patient enrollment in the ADVANCE Trial, a head-to-head randomized ...
October 26, 2022 — Thousands of people have new hope for treatment of thoracic aortic arch disease and University ...
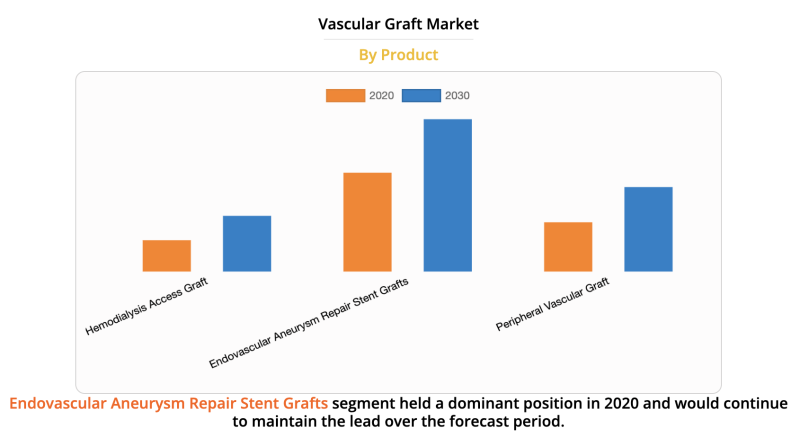
The global vascular graft market size was valued at $4,993.64 million in 2020, and is projected to reach $8,138.68 ...
May 4, 2022 – Seeking to bolster its development of a biocompatible graft that promises to reshape the future of cardiac ...
January 17, 2022 – The Vascular Care Group (TVCG) announced Stephen J. Hoenig M.D., successfully completed a ...
November 22, 2021 — The Minneapolis Heart Institute Foundation (MHIF) announced the publication of research showing ...
January 31, 2020 – The Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) released new reporting ...
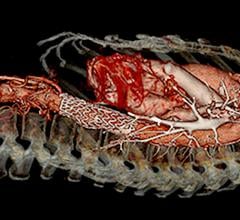
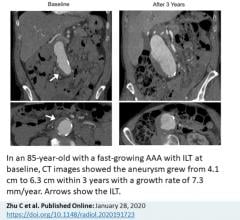
January 29, 2020 – The presence of a blood clot on the wall of the aorta in people with abdominal aortic aneurysms (AAA) ...
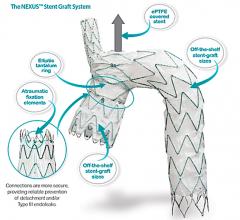
November 7, 2019 — The Endospan Ltd. Nexus aortic arch stent graft is a CE mark–approved, off-the-shelf system for ...
October 9, 2019 — Medtronic plc announced it has received Breakthrough Device designation from the U.S. Food and Drug ...
October 8, 2019 — PQ Bypass Inc. announced it has received full approval of its investigational device exemption (IDE) ...
July 10, 2019 — W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable ...





 January 31, 2024
January 31, 2024