November 14, 2012 — Medtronic Inc. announced that the U.S. Food and Drug Administration (FDA) has approved the company’s ...
Stent Grafts
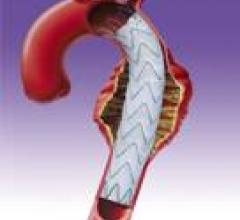
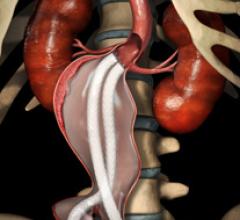
Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
November 9, 2012 — The U.S. Food and Drug Administration (FDA) recently cleared Bolton Medical’s Relay Thoracic Stent ...
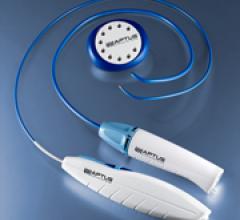
October 4, 2012 — Aptus Endosystems Inc., a medical device company developing advanced technology for endovascular ...
September 21, 2012 — Endologix Inc., developer and marketer of treatments for aortic disorders, announced earlier this ...
July 9, 2012 — Endosystems Inc., a medical device company developing technology for endovascular aneurysm repair (EVAR) ...
June 22, 2012 –– The U.S. Food and Drug Administration (FDA) recently selected a stent graft being developed by ...
June 8, 2012 — Medtronic Inc. announced the U.S. launch of the Endurant II AAA Stent Graft System, which recently ...
April 20, 2012 — VasoStitch will be featuring its technology at EuroPCR, Europe’s largest interventional cardiology scie ...
February 16, 2012 — Aptus Endosystems Inc. a medical device company developing advanced technology for endovascular ...
January 18, 2011 – Medtronic Inc. announced the CE mark and international launch of the Endurant II AAA stent graft ...
January 17, 2012 — The U.S. Food and Drug Administration (FDA) expanded the approved usage for an endovascular graft ...
December 22, 2011 — TriVascular Inc. announced the first Canadian patients treated with its Ovation Abdominal Stent ...
December 14, 2011 — Aptus Endosystems Inc. announced it received 510(k) clearance from the U.S. Food and Drug ...
December 6, 2011 — W. L. Gore & Associates, Inc. (Gore) has received U.S. Food and Drug Administration (FDA) approval ...
November 23, 2011 – Vascular occlusions are blocked blood vessels caused by a blood clot in an artery. Blocks in the ...


 November 14, 2012
November 14, 2012