November 9, 2012 — The U.S. Food and Drug Administration (FDA) recently cleared Bolton Medical’s Relay Thoracic Stent Graft with Plus Delivery System.
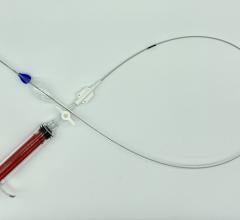
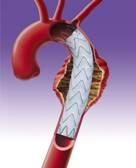
The Relay is an endovascular stent graft used to repair fusiform aneurysms or saccular aneurysms/penetrating ulcers of the aorta in the chest. The stent graft is made of a fabric tube supported by a metal framework. Each endovascular stent graft is compressed into the end of a long, thin, delivery catheter.
A fusiform aneurysm is a type of thoracic aortic aneurysm that has a varying diameter and length and typically involves all sides of the diseased vessel. A saccular aneurysm is a type of aneurysm that is circular in shape and typically involves only one side of the diseased vessel. Saccular aneurysms are often associated with penetrating ulcers. A penetrating ulcer is a weak area of the aorta that causes one side of the diseased vessel to bulge or expand. Unlike a saccular aneurysm, a penetrating ulcer does not go completely through the first layer of the aorta.
The delivery catheter containing the endovascular stent graft is inserted into an artery in the groin through a small incision in the skin. It is carefully guided within the artery into the chest to bridge the site of the aneurysm or penetrating ulcer in the aorta. The endovascular stent graft is then released (deployed) in the aorta where it self-expands to the diameter of the aorta to seal off the aneurysm or penetrating ulcer and relines the artery wall.
The Relay Thoracic Stent Graft with Plus Delivery System can be used in place of invasive open surgery in patients who have a fusiform aneurysm or saccular aneurysm/penetrating ulcer of the aorta in the chest.
For more information: www.boltonmedical.com


 September 18, 2025
September 18, 2025