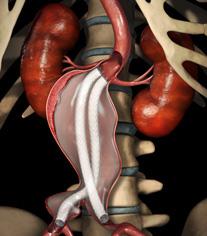
September 21, 2012 — Endologix Inc., developer and marketer of treatments for aortic disorders, announced earlier this month it received CE mark for the current version of the Nellix EndoVascular Aneurysm Sealing System for the treatment of patients with abdominal aortic aneurysms (AAA).
Nellix is a new endovascular aneurysm sealing (EVAS) system designed to simplify endovascular procedures, treat a broader range of patients and provide enhanced clinical outcomes. The Nellix system is not approved in the United States for either investigational use or commercial sale.
Endologix is currently implementing a few enhancements to the Nellix System, intended to further optimize the device for commercialization. When complete, the company will submit these enhancements to its notified body, with expectations of gaining CE mark approval for the commercial version of the device and beginning a limited market introduction of the enhanced system in Europe by the end of the second quarter 2013.
John McDermott, Endologix president and CEO, said, "Nellix has the potential to treat more AAA patients and get better clinical outcomes than any other device for the endovascular repair of AAA. Receiving CE mark for the current version of the system is an important regulatory milestone, and we look forward to providing this technology to physicians and their patients in 2013."
For more information: www.endologix.com


 April 26, 2023
April 26, 2023 









