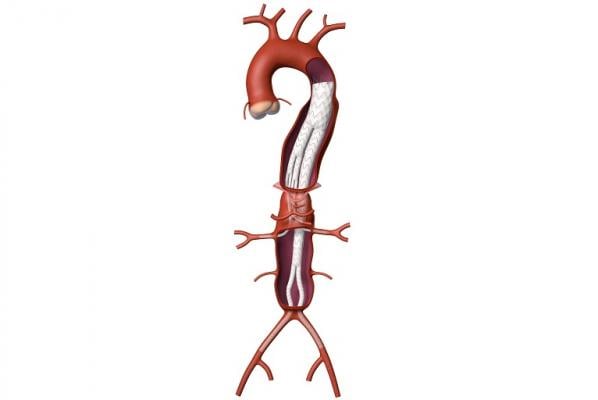
October 9, 2019 — Medtronic plc announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its Valiant TAAA Stent Graft System for minimally invasive repair of thoracoabdominal aortic aneurysm (TAAA). A TAAA is a complex condition causing a bulging of the aorta, which extends from the chest down into the abdomen.
The FDA Breakthrough Device Program is intended to help patients receive more timely access to breakthrough technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. Under the program, the FDA will provide Medtronic with priority review and interactive communication regarding device development and clinical trial protocols, through to commercialization decisions.
The Valiant TAAA is currently being evaluated in the U.S. within five physician-sponsored investigational device exemption (PS-IDE) trials for treatment of TAAA. The system under evaluation in these trials was developed in collaboration with Patrick Kelly, M.D., a vascular surgeon and inventor specializing in complex vascular disease who leads Sanford Health Commercialization. The Valiant TAAA is intended to offer an off-the-shelf endovascular solution with a size matrix to enable broad patient applicability for one of vascular surgery’s most difficult pathologies.
“Breakthrough designation from the FDA means that we will be able to deliver this much needed treatment to patients sooner than expected. With an open surgery mortality rate of 25 percent, it is critical that we deliver for this unmet patient need,” said Murray Shames, M.D., professor and chief division of Vascular Surgery at the University of South Florida Morsani School of Medicine and an investigator for the Valiant TAAA. “Physicians and industry must continue to innovate and provide hope for those with challenging disease states.”
TAAA typically involves the branch arteries that supply blood to multiple internal organs and represents about 15 percent of all thoracic aneurysms. The standard of care is complex open surgery, which is associated with high morbidity and mortality, and 40 percent of patients are not considered candidates for treatment.
The Valiant Navion LSA branch thoracic stent graft system received breakthrough designation from the FDA in May 2019 to allow for more timely delivery of treatment options for patients in need of left subclavian artery (LSA) coverage during thoracic endovascular aortic repair (TEVAR).
Valiant Navion LSA is based on Valiant Mona LSA investigational device and the FDA-approved Medtronic Valiant Navion stent graft system. Like Valiant Navion, the off-the-shelf, single branch device leverages a low-profile design, which may further enable patient applicability and access. The branch cuff is intended to replicate the natural anatomy of the LSA to maximize seal and patency.
For more information: www.medtronic.com


 November 14, 2025
November 14, 2025 









