January 7, 2019 — Endologix Inc. announced that in order to ensure optimal outcomes for patients, unrestricted sales and use of the Nellix System will cease immediately, and the product will only be available for use under clinical protocol with pre-screened patients that adhere to the current indications.
“We monitor the performance of the Nellix System through clinical trials, our complaint monitoring system, physician interaction and available publications,” said Matt Thompson, M.D., chief medical officer of Endologix. “Our independently adjudicated data from the EVAS1 IDE clinical trial indicates that the Nellix System has performed well when used consistent with the current indications. However, data from recent Nellix publications leave us concerned that outcomes are suboptimal when the system is used outside current instructions for use.”
To ensure optimal clinical outcomes, the Nellix System will, for the foreseeable future, only be available for use under clinical protocol with pre-screened patients that adhere to the current indications. All cases will be pre-screened by a physician panel and supported by Endologix clinical specialists to ensure adherence to protocol. Compassionate use requests will be reviewed in accordance with the process established by the company and associated national competent authorities. The existing inventory will be voluntarily recalled. These actions are described in a Field Safety Notification (FSN) issued Jan. 4, 2019.
This decision is one of several actions taken by Endologix following a new management mandate in August 2018 to ensure the most appropriate use of each of its devices and is in alignment with a recent publication by the European Society for Vascular Surgery (ESVS). Endologix has been in contact with regulatory authorities regarding the Nellix System recall and related matters to help ensure patient safety and continued appropriate access to the Nellix System.
Endologix refined the technical procedure of EVAS and voluntarily and proactively delivered clinical practice updates and advisories regarding use of the Nellix System through a series of FSNs developed in collaboration with regulatory authorities worldwide. Nevertheless, Endologix has determined that off-label use is occurring at what it calls an unacceptable level, with the consequence of sub-optimal results. The company is taking action to ensure optimal outcomes for patients.
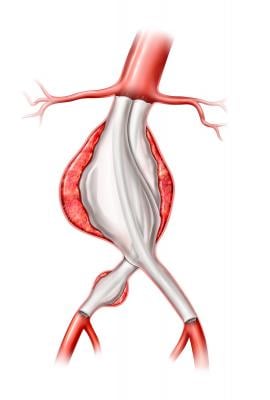
EVAS therapy was designed to overcome the durability issues of conventional endovascular aneurysm repair (EVAR) that have led to high rates of aneurysm-related mortality when compared with surgical interventions.
For more information: www.endologix.com


 September 18, 2025
September 18, 2025 









