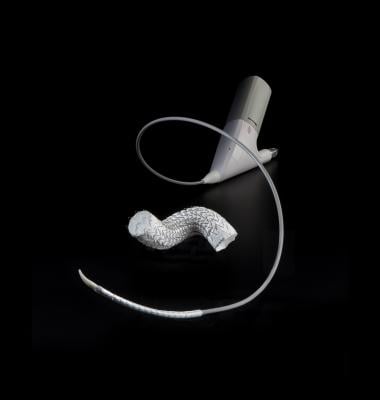
July 10, 2019 — W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable Thoracic Stent Graft with Active Control System. The successful procedure was performed by William Jordan, M.D., chief of the Division of Vascular Surgery and Bradley Leshnower, M.D., cardiothoracic surgeon, at the Emory University School of Medicine in Atlanta. This first case follows the recent U.S. Food and Drug Administration (FDA) approval for this new device.
The Gore Tag Conformable Thoracic Stent Graft with Active Control System delivers new levels of control in the endovascular repair of aneurysms, transections and Type B dissections of the thoracic aorta, according to the company. A key feature of this device is a delivery system that provides controlled, two-stage deployment, with primary deployment to an intermediate diameter and a secondary deployment to full diameter. In addition to the staged deployment, a lockwire keeps the stent graft attached to the catheter through the procedure, enhancing the physician’s control. The device also features a full 2 French reduction in profile over previous designs across 10 device sizes.
“The two-stage deployment provided by the Gore Tag Conformable Thoracic Stent Graft with Active Control System allows for continuous blood flow, and it gives me multiple opportunities to visualize and refine graft placement for accurate placement and peace of mind,” said Jordan. “The new delivery system gives me added precision in placement and angulation so I can take full advantage of the conformability of the Gore device and confidently provide a minimally-invasive solution to more patients, even in patients with complex anatomy.”
The device received CE Mark in 2017. At 30-day follow-up, it demonstrated 100 percent successful deployment and zero type IA or type III endoleaks, fractures, device compressions or ruptures, according to real-world data from the post-market European observational, single-arm SURPASS registry. No device-related issues were reported by 98.4 percent of registry participants, and 97.2 percent of registry subjects were reported free from serious access complications. Further, 98.4 percent of registry participants reported improved proximal wall apposition at procedural completion, and 92.9 percent reported no rapid pacing was used.
For more information: www.gore.com


 November 14, 2025
November 14, 2025 









