June 6, 2014 — Continuing to expand its portfolio of endovascular solutions, Medtronic Inc. announced that two recently ...
Stent Grafts
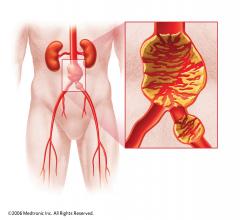
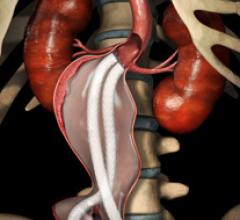
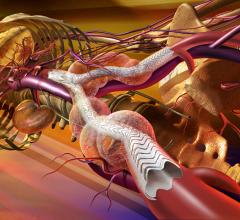
Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
June 2, 2014 — As more endovascular medical programs adapt simulation as an essential part of their training curriculum ...
May 5, 2014 — W. L. Gore & Associates (Gore) announced that Himanshu Patel, M.D. and David Williams, M.D. at The ...
March 6, 2014 — W. L. Gore & Associates’ Gore Excluder Iliac Branch Endoprosthesis is a complete, fully engineered ...
June 24, 2013 — Aptus Endosystems Inc. announced that it received 510(k) clearance from the U.S. Food and Drug ...

May 20, 2013 — Patients with ruptured abdominal aortic aneurysms (AAA) are more than twice as likely to survive if they ...
April 8, 2013 — W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new ...
December 21, 2012 — Despite earlier signs that a less-invasive surgery is safer and better than “open” operations to ...
November 30, 2012 — TriVascular Inc. announced premarket approval (PMA) of the Ovation Abdominal Stent Graft System by ...
Mercy Hospital in Chicago has developed a successful hybrid cath lab program where various specialties work together for ...
November 20, 2012 — W. L. Gore and Associates has announced the commercial availability of its new 10-cm configuration ...
November 19, 2012 — New endovascular grafting techniques to repair tissue following abdominal aortic aneurysm rupture ar ...


 June 06, 2014
June 06, 2014