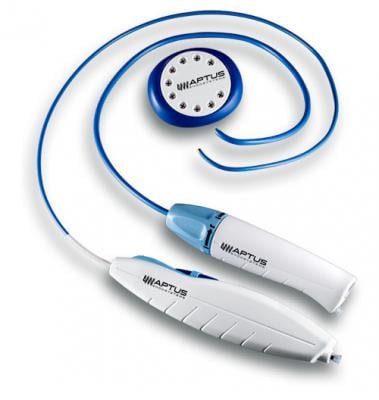
June 24, 2013 — Aptus Endosystems Inc. announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 28 mm Tip Reach Heli-FX Guide. A line extension of the original Heli-FX System, the new product enhances treatment of wide neck abdominal aortic aneurysms (AAA). The announcement comes ahead of this week’s Society of Vascular Surgery (SVS) Vascular Annual Meeting in San Francisco where the company will be launching this device as well as the recently approved Heli-FX Thoracic System.
Aortic aneurysms are an enlarged and weakened section of the aorta, the main artery carrying blood from the heart, which can be lethal if left untreated. Each year, an estimated 200,000 people in the United States and 100,000 people in Europe are diagnosed with AAA. In endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR), an alternative to open surgical repair of aortic aneurysms, a minimally invasive catheter-based system is used to implant a metal and fabric endograft to isolate blood flow away from the aneurysm to prevent potential rupture and death. The innovative helical anchor technology of the Heli-FX system enables independent endograft fixation and sealing, and is designed to replicate hand suturing performed during open surgical repair of aneurysms.
The Heli-FX system can be used during primary EVAR procedures to enhance an endograft’s inherent fixation and sealing mechanisms. Doing so can potentially improve the long-term durability of the aneurysm repair. The system can also repair endovascular grafts that have developed endoleaks, migrated away from the implant site, or are at risk of developing these complications, which are often seen after EVAR. In such cases, augmented fixation and/or sealing is required to regain or maintain effective aneurysm exclusion. The 28mm Heli-FX Guide is a line extension of the original 22 mm Heli-FX Guide and facilitates the precise positioning and implantation of the helical EndoAnchors in aortic necks up to 32mm in diameter.
“The longer reach 28 mm guide gives physicians a greater ability to implant anchors and improve the long term durability of EVAR. This will be a useful addition to the Heli-FX system especially when treating difficult aortic anatomy,” said William P. Jordan, M.D., chief, section of vascular surgery at the University of Alabama Hospital, Birmingham, Ala.
For more information: www.aptusendosystems.com


 September 18, 2025
September 18, 2025 









