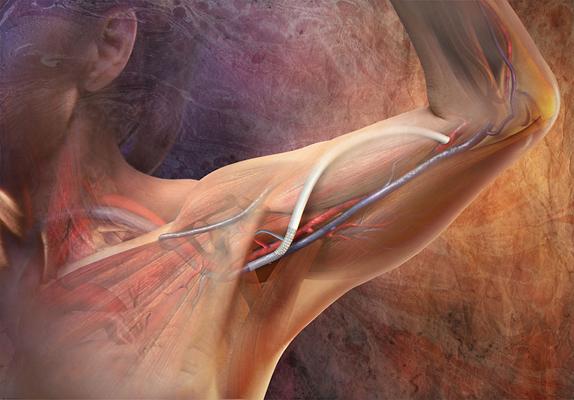
November 20, 2012 — W. L. Gore and Associates has announced the commercial availability of its new 10-cm configuration of the Gore Hybrid Vascular Graft. The new configuration allows for access to deeper vessels, which expands the treatable patient population and offers exceptional ease of use for physicians performing vascular procedures.
The Gore Hybrid Vascular Graft is indicated for use as a vascular prosthesis for replacement or bypass of diseased vessels in patients suffering occlusive or aneurysmal diseases, in trauma patients requiring vascular replacement, for dialysis access or for other vascular procedures.
The new configuration features a 10 cm durable Nitinol Reinforced Section that is double the length of the existing 5 cm Gore Hybrid Vascular Graft. The longer extension allows physicians to access even deeper vessels and create new access sites in anatomical locations that would have otherwise been abandoned, preserving the number of access sites available throughout a patient’s long-term therapy.
As with the original Gore Hybrid Vascular Graft, the 10 cm configuration is highly versatile, providing a streamlined solution for several critical vascular procedures.
John R. Ross, M.D., general surgeon, Regional Medical Center in Orangeburg, S.C., recently performed the first implants of the new 10 cm device for dialysis access. “The new 10 cm Nitinol Reinforced Section of the Gore Hybrid Vascular Graft extends the value of the device by providing minimally invasive access to those vessels that may not have been reachable without major surgery,” Ross said. “This preserves valuable real estate and provides an ability to revise sites that otherwise would have been abandoned. This is an especially important new option for the dialysis access population.”
Physicians Syed Hussain, M.D., Jen Ash, M.D., and Nabeel Rana, M.D., Division of Vascular and Endovascular Surgery, HeartCare Midwest Department of Surgery, University of Illinois College of Medicine in Peoria, Ill., performed the first arterial bypass procedure in the lower limb using the 10 cm configuration of the Gore Hybrid Vascular Graft. “By integrating a longer Nitinol Reinforced Section into the design of the new Hybrid graft, we are able to access even more difficult-to-reach vessels from more proximal sites, thereby expanding our patients’ treatment options,” Ash said.
The Gore Hybrid Vascular Graft also offers several advantages for visceral and renal bypasses, noted Jean Bismuth, M.D., Vascular Surgeon at The Methodist Hospital in Houston, Texas. “This device provides a distinct advantage in renal bypasses by allowing for a rapid and reliable anastomosis, thereby minimizing renal ischemia,” Bismuth said. “Additionally, in visceral and renal bypasses, it permits the surgeon to address the anastomosis and atherosclerotic disease of the vessel simultaneously, optimizing flow.”
The Gore Hybrid Vascular Graft combines several trusted Gore technologies. The expanded polytetrafluoroethylene (ePTFE) vascular prosthesis has a section reinforced with nitinol, which is partially constrained to allow for easy insertion and deployment into vessels that are difficult to reach or in challenging anatomical locations. It is the only combination graft of its kind that incorporates Carmeda BioActive Surface (CBAS Surface) with covalently bonded heparin, resulting in a proven thromboresistant surface.
The Gore Hybrid Vascular Graft was launched in the United States in May 2011 and in Europe in August 2012. It was recently named the Gold Winner in the Implant and Tissue-Replacement Products category of the 2012 Medical Design Excellence Awards. Since commercialization, more than 3,000 successful implants for dialysis access and other vascular procedures have been recorded.
“The new 10 cm Gore Hybrid Vascular Graft demonstrates our commitment to providing physicians with innovative technology that can expand treatment options and improve patient outcomes worldwide,” said Chuck Biggerstaff, associate with the Gore Venous Access Business.
For more information: www.goremedical.com


 September 18, 2025
September 18, 2025 









