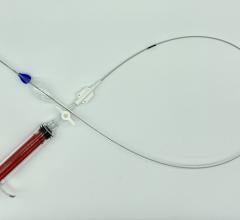
December 6, 2011 — W. L. Gore & Associates, Inc. (Gore) has received U.S. Food and Drug Administration (FDA) approval for new diameter sizes of the contralateral leg component of its Excluder AAA Endoprosthesis. The device, indicated for treatment of abdominal aortic aneurysm (AAA), is now available in 23 mm and 27 mm sizes. The new diameter devices provide physicians with the ability to repair AAAs in a wider range of anatomies eligible for minimally invasive endovascular AAA repair.
“By adding new diameter options to the Gore Excluder device, patients with large iliac arteries can now be treated with fewer components. This will simplify the EVAR (endovascular aneurysm repair) procedure for these patients, widen its applicability, and reduce its costs,” said Michel Makaroun, M.D., professor and chair, division of vascular surgery, University of Pittsburgh School of Medicine.
The Excluder is an endovascular stent-graft that seals off the aneurysm and creates a new path for blood flow. The device is inserted through a small incision in the patient’s leg using a catheter-based delivery technique. Once the physician has positioned the graft in the diseased aorta, the C3 Delivery System uniquely and intuitively enables repositioning of the stent-graft. The ability to reposition the device may minimize complications that could occur if the graft needs to be moved after the initial deployment.
The device is the result of collaboration between EVAR physicians and Gore engineers. With more than 135,000 devices distributed worldwide, the Excluder is characterized by its low profile, flexible on and off catheter characteristics, active infrarenal fixation and strong clinical data. In addition, it is supported by a highly rated clinical support team, a comprehensive educational offering, and Gore’s community awareness programs.
For more information: www.goremedical.com


 September 18, 2025
September 18, 2025