April 2, 2015 — Seventeen-year-old Ariana Smith recently became the first patient at the Children's Hospital of Michigan, and the first in Michigan, to benefit from a revolutionary 3-D printed heart model to treat a very large, complex aortic aneurysm.
In November, 2014, Ariana's mom Jacqueline Foster decided to have her four children undergo an electrocardiogram (EKG) as a precaution since one of her sons had a heart murmur.
When testing revealed Ariana — an active teen who enjoys cheerleading and volleyball — may have potential abnormalities in her aorta, she was referred to the Children's Hospital of Michigan, a part of the Detroit Medical Center (DMC). She received a cardiac catheterization so that doctors could diagnose and potentially treat her condition.
What looked to be a relatively routine procedure turned into a major turn of events when it was discovered that Ariana had a huge aortic aneurysm complicated with a tortuous aorta that has a distorted shape or path. This very dangerous condition could take Ariana's life in the future.
"We were devastated to hear this news. Ariana had no symptoms. I understand an aneurysm is considered a silent killer. We hoped and prayed that everything would turn out ok," Jacqueline said.
"Ariana's condition was extensively discussed with our pediatric cardiovascular surgeons and cardiologists in order to provide the best treatment and outcome. Surgical therapy was a high-risk operation for her age and anatomy which could lead to possible complications with her aorta," said Daisuke Kobayashi, M.D., a cardiologist on staff at the Children's Hospital of Michigan, who managed her treatment and care.
Heart specialists at the Children's Hospital of Michigan started exploring the possibility of treating Ariana using covered stents to expand the artery and block the aneurysm to prevent it from rupturing.
Children's Hospital of Michigan is one of only a few hospitals in the country participating in a research study called the COAST II trial (COarctation of the Aorta Stent Trial). Sponsored by the NuMED company, this U.S. Food and Drug Administration (FDA)-approved study uses stents covered with a special lining for the treatment of aortic narrowing and aneurysms. After the stent is placed in the aorta, the lining or covering becomes the blood vessel wall and blocks blood from entering the aneurysm.
The use of covered stents can treat both the obstruction and the aneurysm. However, this very delicate procedure can cause major complications if not performed well.
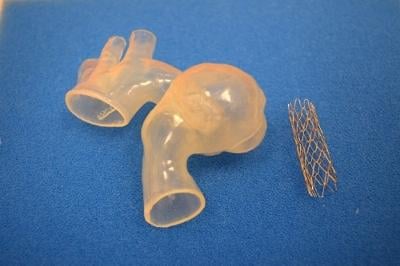
Due to the complexity of the procedure, a team of cardiologists on staff — including Kobayashi, Richard Humes, M.D., Thomas Forbes, M.D. and Daniel Turner, M.D. — collaborated on the use of a revolutionary tool using computed tomography (CT) to generate 3-D printed models of Ariana's heart.
The model was developed by Materialise, based locally in Plymouth, Michigan. Through their Mimics Innovation Suite software and HeartPrint services, Materialise was able to transform Ariana's CT scan into a 3-D printed, life-like replica of her aorta, enabling the cardiology team to precisely plan treatment and practice the intervention prior to the procedure. This allowed the team of doctors to identify potential issues they might find and precisely pinpoint the placement of the stent without surprises.
Turner says in Ariana's case, the 3-D printed model was important for more than just diagnosing and visualizing the problem.
"Using the 3-D printed model of Ariana's aorta, we performed a 'practice-run' or simulation in the cath lab, where we actually placed a stent into the model. This allowed us to precisely plan the procedure and see how the stent responded in her unique and tortuous anatomy. Then, when we performed Ariana's actual procedure, we had a good idea of how it was going to go," he said.
Normally, Ariana's case would require a surgical procedure, with higher associated risk, more discomfort, a longer hospital stay and more time missed from school. With treatment in the catheterization lab, Ariana spent just one night in the hospital and was home the next day.
"We anticipate that Ariana will not require surgery to treat this condition. Dr. Kobayashi will follow her closely in the office. Most importantly, this experience will allow us to treat future patients more safely with the use of 3-D printing technology. This is only the beginning," Turner said.
As for Ariana, after the procedure she enjoyed pancakes, left the hospital after just one day, went back to school after one week and plans to go shopping for prom.
"We are so thankful to God and all of the nurses and doctors at the Children's Hospital of Michigan. Ariana is doing great and we would not have had her treatment anywhere else," Jacqueline said.
For more information: www.childrensdmc.org


 April 26, 2023
April 26, 2023 









