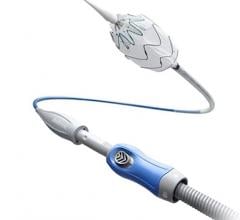
May 25, 2022 — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance for its ŌNŌ retrieval system, the first major advance in endovascular bailout technology in decades. This comes at a time when endovascular closure procedures are rapidly expanding in scope and complexity around the world.
“The FDA clearance of the ŌNŌ is a significant milestone for safety innovation in catheter- based therapies,” said Matthew J. Gillespie, M.D., co-founder of ŌNŌCOR. “I’ve watched the scope and complexity of endovascular devices and procedures increase rapidly over the last 20 years and have been struck by the absence of an equal advancement in rescue technologies. The ŌNŌ was invented to help interventionalists ensure the promise of safety that they make to their patients on a daily basis.”
Catheter-based closure procedures, which are touted as safer alternatives to open-heart surgical procedures, are now routinely performed in cardiac catheterization labs and endovascular suites. Interventions such as patent foramen ovale (PFO) closure, left atrial appendage (LAA) occlusion, atrial septal defect (ASD) closure, ventricular septal defect (VSD) closure, and patent ductus arteriosus closure, among others, are performed tens of thousands of times per year globally.
Though generally safe, these complex procedures carry unavoidable risks. Problems such as device misplacement, embolization, or improperly functioning implants can necessitate a rescue procedure for device removal. The ability to safely retrieve and remove a wayward device is paramount to ensuring the promise of safety currently ascribed to these proliferating endovascular therapies.
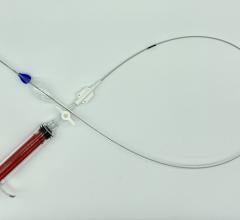
The ŌNŌ is a novel device designed to receive, align, compress, and remove suboptimal or embolized devices from the vascular system through 12Fr or larger sheaths. ŌNŌ was designed to be intuitive to use and is compatible with commercially available vascular sheaths and endovascular snares.
“We believe that every interventionalist performing a catheter-based procedure should have an ŌNŌ device at hand for the inevitable occasion when a problem occurs,” said Mark Piper, CEO of ŌNŌCOR. “Mitigating the consequences of even rare events should be paramount for every physician.”
ŌNŌCOR plans for commercial launch of the ŌNŌ across the United States beginning in 2022.
For more information: www.onocorvascular.com


 September 18, 2025
September 18, 2025