February 18, 2022 – Front Line Medical Technologies, Inc. today announced the expanded availability and distribution of ...
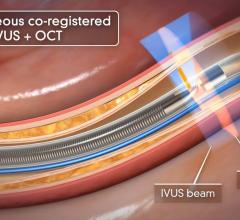
February 18, 2022 — GE Healthcare has announced that it has received approval from the European Medicines Agency (EMA) ...
We are launching something new—a Clinical Case of the Month—and we want you to contribute! Do you have a clinical case ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
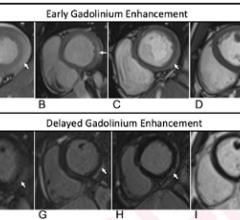
February 17, 2022 — While cardiac imaging shows that COVID-19 vaccine–associated myocarditis has a similar pattern as ...
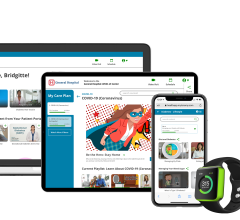
February 17, 2022 – Mytonomy, known for raising the bar in video-based patient-engagement content and platform, is ...
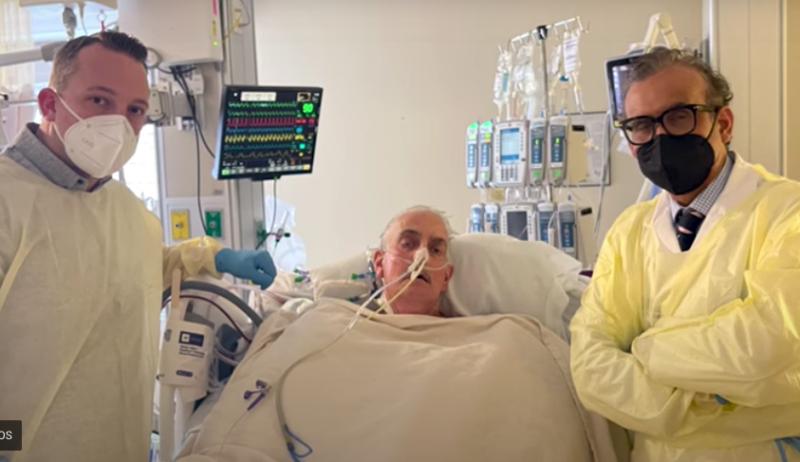
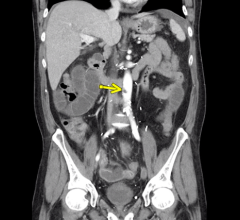
In January, The University of Maryland School of Medicine (UMSOM) and the University of Maryland Medical Center (UMMC) ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
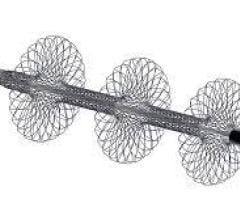
February 16, 2022 – Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, has announced ...
February 16, 2022 – Cardiology is an inherently data-dependent and data-driven practice. Patient medical history records ...
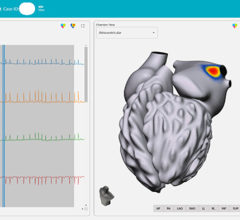
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
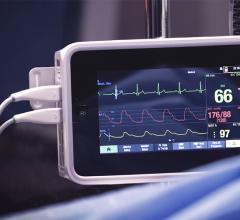
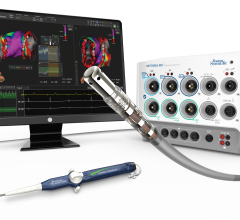
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

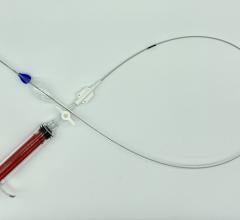
 February 18, 2022
February 18, 2022