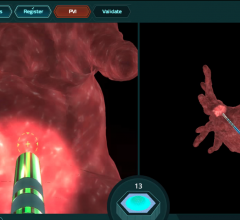
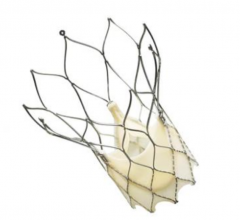
June 7, 2018 — The first clinical cases have been completed where the Emboliner Embolic Protection Catheter was used ...
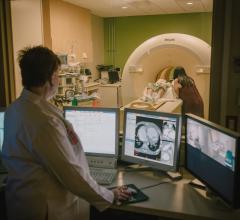
Radiation dose management is central to child patient safety. Medical imaging plays an increasing role in the accurate ...
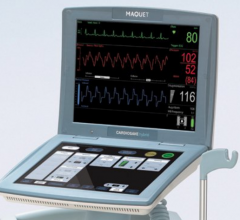
June 6, 2018 — Maquet Datascope Corp. said it is recalling the CardioSave Hybrid Intra-aortic Balloon Pump (IABP) due to ...
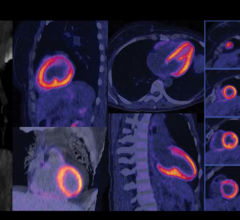
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

June 6, 2018 — Here is a checklist of dose-sparing practices for angiographic X-ray imaging used in the cath lab. This ...
June 5, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Biograph Vision, a new positron emission ...
June 5, 2018 — Philips Healthcare has signed an agreement to acquire EPD Solutions, a provider of image-guidance in ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

June 5, 2018 — The future of cardiovascular care will be transformed by advances in artificial intelligence, digital ...
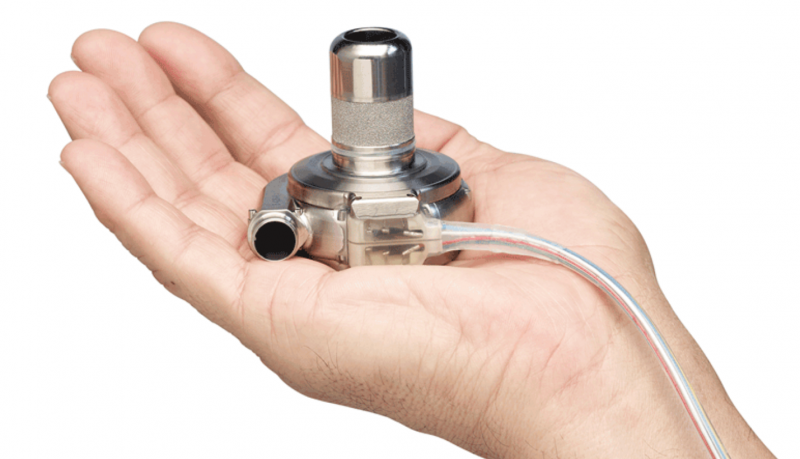
June 4, 2018 — The U.S. Food and Drug Administration said Medtronic initiated a Class 1 recall all 204,017 of its ...
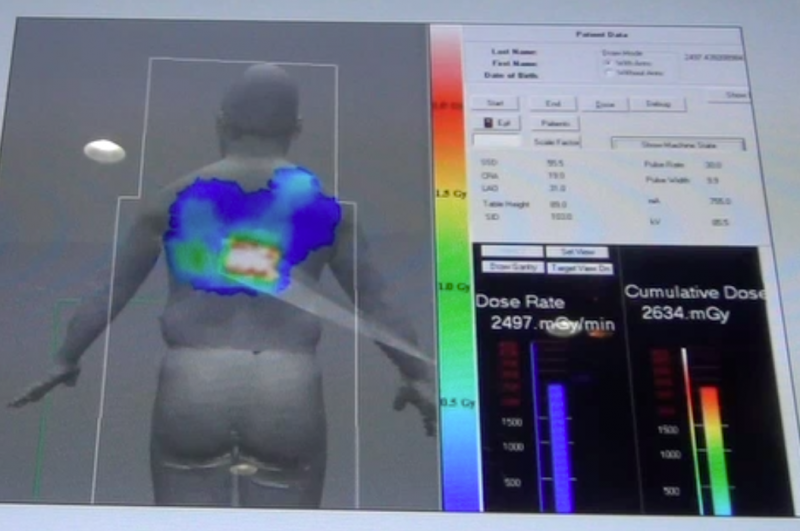
A new consensus document on how to mitigate radiation exposure in cardiac imaging for both patients and staff was issued ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 1, 2018 – Use of the Abbott Portico transcatheter aortic valve replacement (TAVR) therapy was associated with ...
The U.S. Food and Drug Administration (FDA) is issuing a proposed order to reclassify certain radiological medical image analyzers, or computer-aided detection (CAD) devices, from class III to class II devices. This includes CAD devices for mammography breast cancer, ultrasound breast lesions and radiograph lung nodules.

June 1, 2018 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
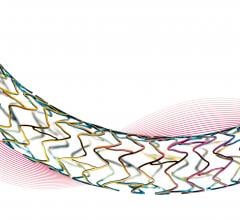
Late-breaking trial results presented at the EuroPCR Congress, May 21-24 in Paris, France, found the Optimax titanium-nitride-oxide (TiNO)-coated Optimax stent was superior to the Synergy everolimus-eluting stent (EES) in acute coronary syndrome (ACS) patients. Results were presented by Pasi Karjalainen, M.D., Heart Center, Satakunta Central Hospital, Pori, Finland.
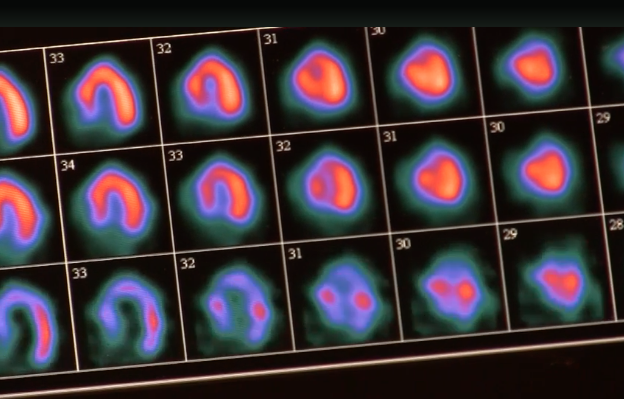
May 31, 2018 — Here is a checklist of dose-sparing practices for nuclear cardiology that was included in a new 2018 ...
Two-year outcome data from the BIO-RESORT randomized controlled trial were presented in a late-breaking clinical trials session at EuroPCR 2018, May 21-24 in Paris, France.


 June 07, 2018
June 07, 2018