February 8, 2018 – ScImage, a leading provider of enterprise PACS solutions, is celebrating 25 years of providing ...
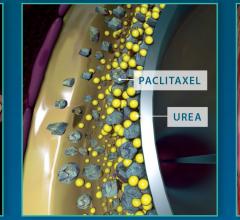
Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug-coated balloon (DCB) with new presentations that demonstrated durable and consistent clinical outcomes in peripheral artery disease (PAD). The new data, presented at the Leipzig Interventional Course (LINC), Jan. 30-Feb. 2, 2018, in Leipzig, Germany, included the two-year results from the MDT-2113 study (IN.PACT SFA Japan) and data from a critical limb ischemia (CLI) subgroup analysis from the IN.PACT Global Study.
Five new angiography systems were launched in 2017 that included improvements to speed workflow and improve both image ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S. commercial launch of new versions of its Expro Elite and Sympro Elite Snares for manipulating interventional devices in peripheral procedures.
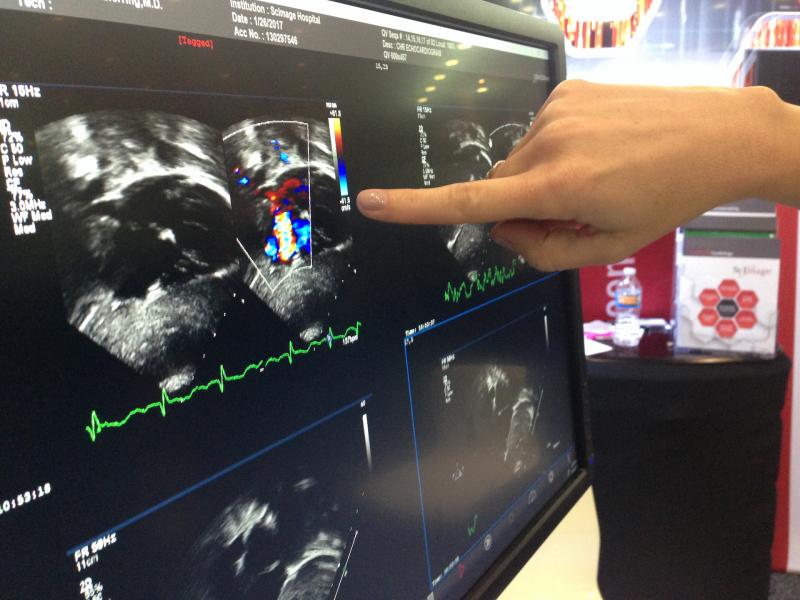
Here is a list of links to recorded session presentations from previous meetings of the American Society of ...
High exposure to radiofrequency radiation (RFR) in rodents resulted in tumors in tissues surrounding nerves in the hearts of male rats, but not female rats or any mice, according to draft studies from the National Toxicology Program (NTP). The exposure levels used in the studies were equal to and higher than the highest level permitted for local tissue exposure in cell phone emissions today. Cell phones typically emit lower levels of RFR than the maximum level allowed. NTP’s draft conclusions were released as two technical reports, one for rat studies and one for mouse studies. NTP will hold an external expert review of its complete findings from these rodent studies March 26-28.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
February 6, 2018 – Endologix, a developer and marketer of treatments for aortic disorders, announced the completion of ...

Here are several considerations for physicians when choosing endovascular stent grafts used to treat aortic aneurysms ...
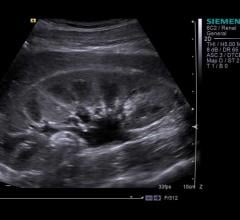
February 5, 2018 — Sacs of fluid in the kidneys may indicate there is also blood vessel damage in the brain and a ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
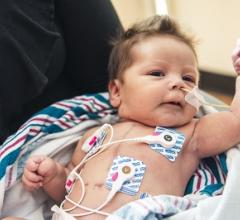
February 5, 2018 — Children's Hospital Los Angeles announced participation in the first-ever clinical trial using stem ...
February 1, 2018 – HeartFlow announced that seven new commercial payers issued positive medical policies covering the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
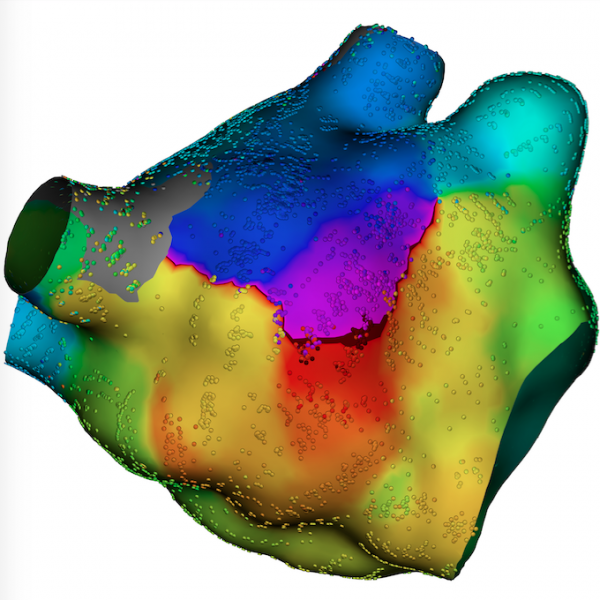
February 1, 2018 –Stereotaxis, who makes innovative robotic technologies for the treatment of cardiac arrhythmias ...
February 1, 2018 — Breast cancer patients may be at increased risk of cardiovascular diseases including heart failure ...
February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...

 February 08, 2018
February 08, 2018