December 6, 2017 — At the American Heart Association (AHA) annual meeting in November, a group of 16 non-partisan ...
December 6, 2017 — Electronic cigarettes are more frequently used by people who recently quit smoking and alcohol ...
December 6, 2017 — Women who develop high blood pressure during pregnancy are more likely to experience heart problems ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 6, 2017 — Older women who do not get enough sleep were more likely to have poor cardiovascular health ...
A tool designed to more accurately predict the risk of heart attack in older patients undergoing non-cardiac surgery works significantly better than traditional risk assessment tools, according to new research from the University of California Los Angeles (UCLA). By having more accurate information, older patients and their physicians can make an informed decision on whether to undergo surgery, UCLA researchers concluded.
Infant deaths from critical congenital heart disease (CCHD) decreased more than 33 percent in eight states that mandated screening for CCHD using a test called pulse oximetry. In addition, deaths from other or unspecified cardiac causes decreased by 21 percent, according to a recent study.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Toshiba Medical, a Canon Group company, introduced its newest magnetic resonance (MR) system, the Vantage Elan/Zen Edition 1.5T, to deliver comfortable, effortless and efficient exams in healthcare facilities of all sizes. The Zen Edition prioritizes intelligent workflow and patient comfort, providing clinical applications to help providers make accurate diagnoses.
Fujifilm Medical Systems U.S.A. Inc. announced the expansion of the company's artificial intelligence (AI) development initiative with entry in the U.S. market. The AI development initiative will harness the power of AI to enhance Fujifilm’s imaging and informatics healthcare Synapse portfolio which includes Synapse PACS (picture archiving and communication system), Synapse Cardiovascular and Synapse VNA (vendor neutral archive), among other solutions. The initiative was on display at the 2017 Radiological Society of North America (RSNA) annual meeting, Nov. 26-Dec. 1 in Chicago.
Toshiba Medical, a Canon Group company, introduced new educational tools and interactive learning resources to help healthcare providers stay current on CMEs and clinical education without impacting busy schedules. The new advancements give clinicians more accessible and mobile-friendly resources and accreditation opportunities that fit into their on-the-go schedules.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
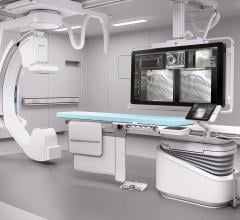
Philips recently announced the results of a comprehensive, independent, two-year study demonstrating the clinical workflow benefits of its next-generation image-guided therapy platform, Azurion. The study investigated nearly 800 patient procedures to evaluate the impact of Azurion at St. Antonius Hospital in Nieuwegein, the Netherlands. The data demonstrated clinicians’ use of Azurion resulted in significant time savings for the hospital, including a 17 percent reduction of the average interventional procedure time, a 12 percent reduction of in-lab patient preparation time, and a 28 percent reduction of post-procedure lab time.
December 4, 2017 — Vital Images unveiled the newest version of Vitrea Advanced Visualization software, the cornerstone ...
Samsung Electronics debuted its OmniTom mobile 16-slice computed tomography (CT) scanner at the Radiological Society of North America (RSNA) 2017 Annual Meeting, Nov. 26-Dec. 1 in Chicago. OmniTom received 510(k) U.S. Food and Drug Administration (FDA) clearance for the U.S. market on Aug. 18 of this year.
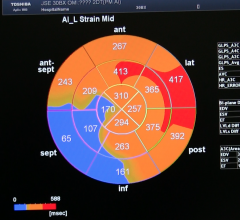
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 25, 2017 — Drinking coffee may be associated with a decreased risk of developing heart failure or having stroke ...
November 25, 2017 — Children and young adults with diabetes may be seven times more likely to die from sudden cardiac ...
November 25, 2017 — Sitting in, or standing close to the charging port of a Tesla electric vehicle did not trigger a ...

 December 06, 2017
December 06, 2017