Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in asymptomatic patients more accurately. Now, providers are using dedicated cardiac ...
Feb. 6, 2026 — Robert Wood Johnson University Hospital (RWJUH), in partnership with Rutgers Robert Wood Johnson Medical ...
Feb. 6, 2026 — Abbott has announced new clinical data from two late-breaking presentations at AF Symposium in Boston ...
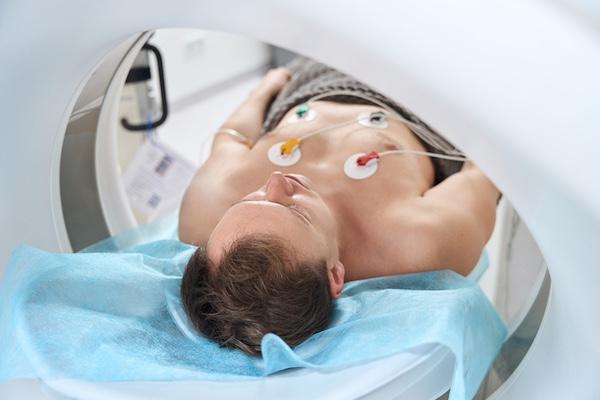
Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Feb. 3, 2026 — The first official summit of the European Association of Percutaneous Cardiovascular Interventions (EAPCI ...
Feb. 3, 2026 — Medtronic plc announced it will exercise its option to acquire CathWorks, a privately held medical device ...
Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Jan. 31, 2026 — The Society of Thoracic Surgeons (STS) has elected Vinay Badhwar, MD, as its 62nd president during the ...
Feb. 2, 2026 — GE HealthCare has announced that Allia Moveo has received U.S. Food and Drug Administration (FDA) 510(k) ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Jan. 28, 2026 — Corify Care has announced a major development in cardiac electrophysiology with the publication of its ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Jan. 27. 2026 — Circle Cardiovascular Imaging Inc. has announced the release of cvi42 v6.4, the latest version of its ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Jan. 27, 2026 — BURL Concepts, a private company developing a rapid, automated and affordable test for evaluating ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Jan. 27, 2026 — A new national study reveals a stark disconnect between Americans’ desire for preventive cardiac ...


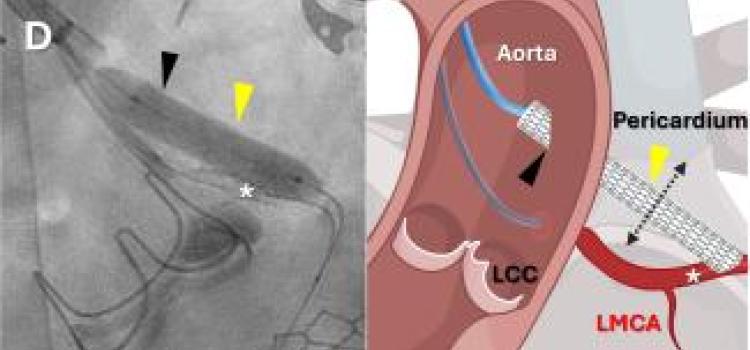

 February 06, 2026
February 06, 2026

















