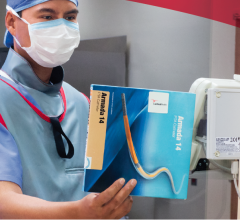
WaveMark's RFID-enabled cabinets store and record all of the high-ticket items for a cath lab and makes real-time inventory information immediately available.
Tight inventory control in the cath and electrophysiology (EP) labs is critical to hospitals' bottom lines due to the expense of the items and their rapid expirations. A new technology showing big returns on investment is radio-frequency identification (RFID) tracking.
Passive RFID inventory tracking cabinet systems can scan inexpensive tags on each item in the cabinet, similar to retail store alarm systems reading passive RFID tags on merchandise as it passes through sensors at the store's exit. These systems can automatically track the inventory of what is in the cabinet, when it is removed, by whom, at what time. The systems can also track expiration dates, which is the biggest area of return.
RFID eliminates manual data entry or bar-code scanning. It can also help solve issues with lost revenues due to items that were used, but someone forgot to enter into the billing system; it helps track exact cost per case and aids vendors in managing inventory. The system can also help track and alert for specific items that are expiring and assist with product recalls.
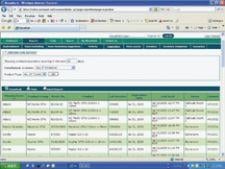
The WaveMark Clinical Inventory Management Solution (CIMS) system uses mobile RFID-enabled storage cabinet carts that can instantly read all the tagged items in the cabinet, such as cardiac stents, balloons and pacemakers. Each product box is tagged with a passive 13.56 MHz RFID tag. The product information can be accessed using a Web-based database.
“We have more than offset the cost (of implementing RFID), and we have definitely saved with this system,” said Dan Scharbach, regional director of invasive heart and vascular services at Providence Health System in Portland, OR.
His lab has been using the WaveMark RFID system for about a year and he said the lab has seen at least a two-fold savings in inventory purchases.
He said the carts are stocked and automatically checked by computer for a complete inventory prior to being unplugged from the wall and its Internet connection and rolled into a cath lab for a procedure. The cart's RFID system is not wireless, so it is not powered during a procedure to ensure there is no electromagnetic interference with medical devices, Scharbach says.
He said the hospital tracks stents, peripheral products, guiding catheters, and neurovascular products used to treat strokes or aneurisms. Generally items tracked are expensive, low-volume use or items with regulatory restrictions. For example, drug-eluting stents cost around $2,500 each and only have a shelf life of about three months. In EP labs, cardiac rhythm devices can range in price from $5,000-$35,000, implantable cardioverter-defibrillator (ICDs) can cost $30,000 or more. He said catheters used in these procedures can run several thousand dollars each.
With the low shelf life and high expense of some of these products, tight inventory control becomes a critical factor in return on investment. Users say RFID allows hospitals to keep lower inventories and a platform to allow vendors and hospitals to track short shelf-life items.
“If you have 2,000 items in your lab, you can imagine it would be difficult to do inventory each month to check for expiration dates,” Scharbach said. “Theoretically, you should never have expired products in your inventory using RFID.”
Scharbach said he knows of one hospital that threw out about $175,000 in cath lab inventory because of past expiration dates. He said RFID has major implications because it can help prevent this level of loss.
Providence Health works with Cordis and purchases many of its cath lab products on a consignment basis. Scharbach explained the company's representative is allowed to access the WaveMark system via the Internet to check inventory levels and expiration dates.
This type of partnering and consignment system could help manufacturers if more cath labs used RFID tracking systems, because the companies could better control their expiration-sensitive inventories and only produce what is needed.
“You can keep a lower inventory because you can track it more accurately. You can reduce inventory to a point with the carts to where they pay for themselves,” Scharbach explained.
Providence Health uses five WaveMark carts in the cardiovascular lab, but Scharbach said plans are in the works to add additional carts because of the cost savings they have made.
Scharbach said specific size stents are ordered for particular patients in advance of their procedures, and the RFID system can also help track these items once they are entered into inventory to ensure they are available for the intended patient.
Some types of stents the hospital stocks can only be used under very specific conditions set by the FDA, such as covered stents. Scharbach said cath labs need to account for their use, and the RFID system helps them keep track of when these items were used.
A lab may only have a couple low-volume use items in stock, so Scharbach explained it is nice to know using the RFID system if two out of three items in stock may have been used over the weekend.
Recording the use of a stent for inventory management is sometimes forgotten during emergencies, but the RFID system will always catch items that were taken out of a cabinet.
“That is very helpful when you have a fast-paced stroke patient who comes in at 3 a.m.,” Scharbach added.
Many cath labs will perform regular, manual inventory counts, which can take hours. With the RFID system, getting an accurate inventory count takes seconds.
“It really saved us a lot of time and effort,” Scharbach said.
He said the hospital has only run into one issue with the system over the past year — people moving the carts without first unplugging them from both the wall and the Internet connection.
“We had to put straps on the walls to remind people to unplug the carts before moving them,” he said.
Other passive RFID cath lab inventory tracking systems are offered by Matrix Product Development Inc. and Mobile Aspects' iRISupply.
Mobile Aspects said its iRISupply system was implemented in the cardiac catheterization and electrophysiology laboratories at King's Daughters Medical Center (KDMC), a 385-bed community hospital in Ashland, KY. KDMC estimated it was incurring an annual expense of $250,000 to $300,000 by inadvertently allowing items to expire on the shelves. After implementing the iRISupply system, Mobile Aspects said the hospital lowered that annual expense to $1,000 to $2,000.
The Mobile Aspects system uses a cabinet with secured doors, which a user must enter a password into a built-in keyboard or swipe an ID through an integral card reader. The cabinet records the time, person and what items they removed or returned. The system will automatically read any items that are returned to the cabinet and re-enter them into inventory. The cabinet’s computer can be integrated with hospital information systems.
Tracking patients in the OR
Several companies offer RFID systems to track patients through the perioperative process in the OR or cath lab. The systems are designed to boost patient safety, patient identification, surgical information verification, allow families to track their family member's progress and to help hospitals increase efficiency and identify workflow issues.
PeriOptimum's PathFinder system instantly communicates the status and location of a surgical patient to medical staff and family members in real time. The status can be viewed on a tracking screen in the waiting room without family members having to bug nurses or make several calls. The system cut phone calls to the surgical department at Mississippi Baptist Medical Center by 60 percent since it was implemented in 2004.
“It has really helped on communications and in reducing the phone calls,” says Kempf Poole, FACHE, director of surgical services, Mississippi Baptist Health in Jackson. “It has reduced phone calls immensely.”
The system allows families to leave the waiting room to visit the cafeteria or gift shop. They can check the status of their family member using a number code to unlock information specific to a patient using a computer kiosk.
“In addition to tracking the physical location of a patient, the RFID tags have a button on them that staff can push to signify a procedure has been done at a particular stage of the perioperative process.
“I know it has helped improve patient safety because it has dramatically improved hand-offs,” Kempf said.
In addition, the hospital used its PeriOptimum RFID system to analyze throughput, optimize scheduling and increase OR capacity by identifying bottlenecks. It also helped prevent issues with staff members arriving late or waiting around for another procedure to be completed. The information can also be tailored to find specific information on particular staff members or particular operating rooms.




 April 28, 2017
April 28, 2017