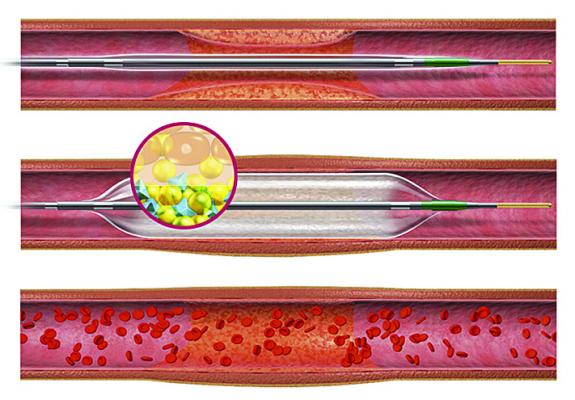
An illustration of the Sequent Please drug-coated balloon.
November 2, 2017 — The treatment of in-stent restenosis (ISR) remains challenging in clinical practice. The DARE (Drug-eluting bAlloon for in-stent Restenosis) multicenter, randomized clinical trial evaluated the performance of the B. Braun Sequent Please drug-eluting balloon (DEB) vs. the Abbott Xience everolimus-eluting stent (EES) in patients with ISR. The study found the DEB was non-inferiority to the drug-eluting stent (DES).
“In this largest trial of DEB versus DES for any ISR to date, the use of a DEB was non-inferior in terms of in-segment minimal lumen diameter at six-month follow-up,” said Jose P.S. Henriques, M.D., Head of the Catheterization Laboratory at the Academic Medical Center, University of Amsterdam in Amsterdam, The Netherlands. “Future research is warranted to investigate the safety and efficacy of the DEB as compared to DES for ISR in adequately powered, large-scale randomized clinical trials.”
A total of 278 patients of whom 56% had DES-ISR were randomized at eight sites to treatment with DEB (N=141) or DES (N=137).
Henriques said the results showed non-inferiority of Sequent Please DEB vs. Xience for any ISR. There was greater acute gain with DES vs. DEB, but this was offset by greater DES late loss at six-month follow-up.
“The DEB appears to be an alternative therapy for any ISR negating the need for additional stent,” Henriques said. “There were no differences in clinical endpoints, including TVR.”
He added that the study results confirm the European guidelines that list DEBs as a Class 1A therapy for in-stent restenosis.
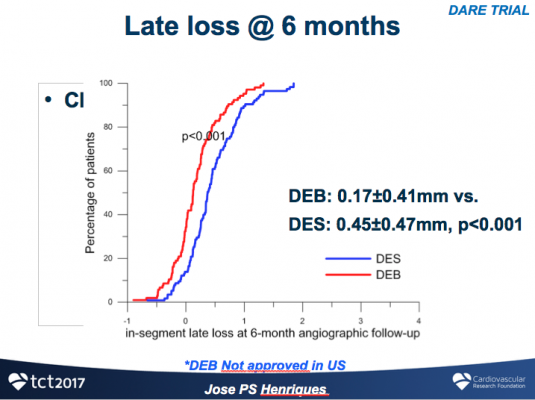
As compared with DEB, DES was associated with larger minimal lumen diameter (MLD) and lower percent stenosis immediately post-procedure (1.84±0.46 vs. 1.72±0.35, P=0.018 and 26±10% vs. 30±10%, P=0.03). Angiographic follow up was completed at 196±53 days in 79% of patients. With respect to the primary endpoint of in-segment MLD at six months, DEB was non-inferior to DES (DEB 1.71±0.51.mm vs. DES 1.74±0.61.mm, P-noninferiority <0.0001), with a lesser degree of late loss compared to DES (0.17±0.41 vs. 0.45±0.47, P<0.001). Target vessel revascularization at 12-month follow-up was similar in both groups (DES 7.1 vs. DEB 8.8%, P=0.65).
The DARE study was an investigator-initiated study funded by the Academic Medical Center - University of Amsterdam and supported by an unrestricted research grant from B. Braun. Dr. Henriques reported receiving an unrestricted research grant from BBraun.
The results of the DARE study were published simultaneously in JACC: Cardiovascular Interventions.
For more information: www.crf.org
Related Content
TCT Announces 2017 Late-breaking Clinical Trial Presentations
Reference:


 June 13, 2024
June 13, 2024 









