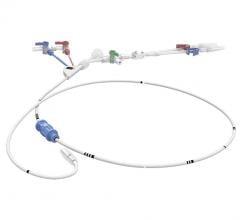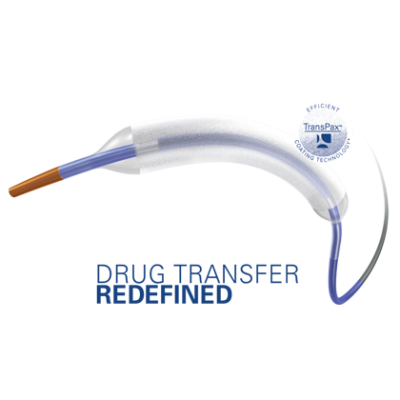
May 20, 2021 — Boston Scientific Corporation announced it has initiated the AGENT IDE trial for the Agent Drug-Coated Balloon (DCB), the first clinical trial in the U.S. to evaluate the safety and effectiveness of a DCB in patients with coronary in-stent restenosis (ISR).
Coronary stenting, in which a stent is inserted into a coronary artery to restore blood flow to the heart, continues to show a substantial improvement in symptoms and quality of life for patients with coronary artery disease. However, in some patients, the stented section of the artery may become obstructed or narrowed by scar tissue, leading to a condition known as ISR. Approximately 10% of percutaneous coronary interventions (PCIs) address this issue and current treatment approaches include the insertion and layering of additional stents, or radiation, both of which introduce potential safety risks for patients.
"While the possibility of ISR remains a challenge for cardiologists, the Agent DCB provides a potential new approach to re-opening the vessel and reducing the risk of ISR reoccurrence over time,” said Principal Investigator Robert W. Yeh, M.D., director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at the Beth Israel Deaconess Medical Center, and associate professor of medicine at Harvard Medical School. “In this new study, we are evaluating a metal-free and radiation-free treatment that could become a safe and more effective long-term alternative for certain patients.”
The AGENT DCB is a percutaneous transluminal coronary angioplasty balloon that is coated with an anti-restenotic drug and delivered via a proprietary coating technology. The balloon is designed to open narrowed vessels and then transfer the drug to the vessel wall. This technology was granted Breakthrough Device designation by the U.S. Food and Drug Administration earlier this year, a status intended to provide patients more timely access to novel devices that may provide a substantial improvement over existing therapies. The AGENT IDE trial is designed to assess the safety and effectiveness of treating ISR with the Agent DCB.
The prospective, randomized trial will compare the Agent DCB to treatment with an uncoated balloon, commonly referred to as “plain old” balloon angioplasty (POBA), in patients with ISR in lesions up to 26 mm in length in a coronary artery 2.0 mm to 4.0 mm in diameter. The study will evaluate the 12-month target lesion failure rate, defined as myocardial infarction related to the target vessel, the need for a revascularization procedure or cardiac mortality. At least 480 patients will be enrolled in the study at 40 U.S. sites.
“We believe the Agent DCB could expand options for physicians managing in-stent restenosis, which can be difficult to treat and frequently reoccurs with conventional treatments,” said Ian Meredith, M.D., global chief medical officer, Boston Scientific. “We are pleased to be leading this study, which may bring this technology to a greater number of patients with coronary artery disease and address the unmet need for a reliable and sustained treatment for their ISR.”
The device has been approved in Europe since 2014 for patients with ISR and previously untreated small vessel coronary disease. The 2018 European Society of Cardiology and European Association for Cardio-Thoracic Surgery Guidelines on Myocardial Revascularization support the use of DCBs with a Class IA recommendation for patients with in-stent restenosis of drug-eluting stents or bare-metal stents.
For more information: www.bostonscientific.com


 June 13, 2024
June 13, 2024