December 12, 2013 — The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee voted favorably Dec. 11 in a 13-1 vote that the benefits of the Watchman
Left Atrial Appendage Closure (LAA) device outweigh the risks. The FDA Panel also voted 13-1 that there is reasonable assurance the device is safe and effective. The FDA will take into account the panel's review in its final decision on approval of the Watchman device. The company expects a decision from the FDA in the first half of 2014.
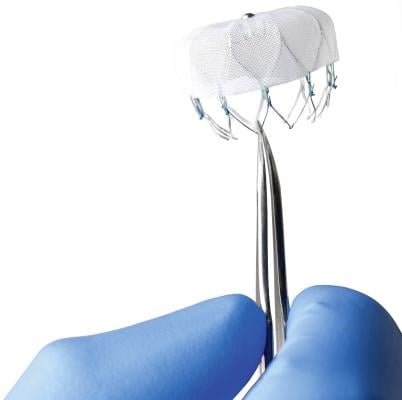
The Watchman is the first transcatheter LAA occluder device to go before the FDA for review. The device is designed to replace warfarin therapy for patients with atrial fibrillation (AF) to prevent blood clots from forming in the LAA. The device is delivered in a cath lab via catheter through a septal wall puncture from the right into the left atrium. The device self expands into the LAA and is anchored by small barbs. It has a fabric covering with facilitates endothelization.
"We are pleased with the outcome of today's panel, which represents an important milestone toward making this innovative technology available to patients with AF at higher risk for stroke who need an alternative to long-term warfarin therapy," said Kenneth Stein, M.D., chief medical officer, cardiac rhythm management, Boston Scientific. "We appreciate the opportunity to present our comprehensive data supporting the Watchman technology and look forward to continuing discussions with the FDA regarding the panel's comments."
The vote of the committee followed a review of clinical data from two randomized control trials, PROTECT AF and PREVAIL, as well as from the CAP (Continued Access Protocol) registry. Watchman is the most studied LAA closure device and the only one with long-term clinical data from 2,000 patients and with almost 4,900 patient-years of follow-up in clinical trials. The Watchman device received CE mark in 2005. In the United States, it is an investigational device, limited to investigational use and not available for sale.
The LAA is a thin, sack-like appendix arising from the heart and is believed to be the source of a majority of stroke-causing blood clots in people with AF. The most common treatment for stroke prevention in patients with AF is blood-thinning warfarin therapy. Despite its proven efficacy, long-term warfarin therapy is not well tolerated by some patients and carries a significant risk for bleeding complications. Due to the drug’s narrow therapeutic window it also requires regular blood test monitoring.
For more information: www.bostonscientific.com
Bottom of Form