
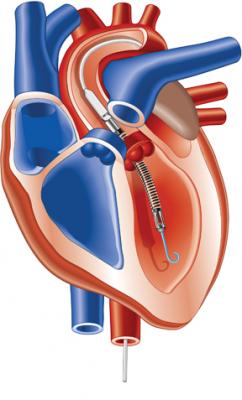
Abiomeds Impella 2.5 ventricular assist device draws blood from the left ventrical and pumps it into the aorta.
There is growing interest in going beyond using ventricular assist devices (VADs) just for end-stage heart failure and expanding use for therapeutic recovery in patients with cardiogenic shock, stage III heart failure and to increase perfusion during acute myocardial infarction (AMI).
VADs are generally used in the sickest patients, many of whom are in the final stages of heart failure. However, occasionally VADs allow the heart to recover on its own, which has stimulated research into other methods to heal the heart. Physicians at some institutions are using VADs as a way to allow the myocardium to rest and recover, while the device takes over some of the work to keep the blood flowing.
With cardiogenic shock and heart failure patients, inotropic drug support, such as dobutamine, is usually used to “whip” the heart to beat faster to increase blood flow and perfusion, said James R. Revenaugh, M.D., FACC, medical director, cardiac catheterization laboratories, Intermountain Medical Center, Murray, Utah. However, he said the drugs overwork the myocardium, contributing further to issues with ischemia or heart failure.
“We really have seen if you are whipping the heart it is much more difficult for it to recover,” Dr. Revenaugh said.
VADs can be used as a replacement for dobutamine to unload the heart in patients who have chronic heart failure with decompression, said Srihari S. Naidu, M.D., director, cardiac catheterization lab, Winthrop University Hospital, Long Island, N.Y. He said the current method to unload these hearts is to put patients on a “dobutamine holiday” and dialysis. He said this usually requires a hospital stay of three days. They are discharged and are fine for a few months, but then return and need a repeat treatment. Dr. Naidu agrees whipping the heart is not the best therapy for a heart that is already straining. He said the drug also has potential side effects of causing arrythmias/tachycardias.
Theoretically, a VAD could shorten the patient’s hospital stay because it’s more efficient and less of a shock to the patient’s heart, Dr. Naidu said. He said VADs increase blood flow without the heart working harder, allowing the myocardium to rest. “We are very interested in its potential,” he said. “It’s a lot safer going forward (than drug treatment).”
Dr. Revenaugh had patients on VAD support for three or four days, took them off and found their hearts had recovered. But, he said the key factor seems to be the patient’s age.
“I am impressed how hearts can recover, mainly in young people,” he said. “The younger the person, the more likely they are to recover.”
He pointed to two cases in his lab where younger patients recovered thanks to VAD therapy. A 19-year-old woman with ventricular fibrillation and prolonged CPR was brought into the lab with an ejection fraction of less than 10. Dr. Revenaugh said her heart was in shock and a VAD was placed to increase flow and unload the heart. He said the VAD helped her recover and today she is leading a normal life and has an ejection fraction of more than 45 percent.
In another case a 37-year-old patient with heart failure was hooked up to a cardiopulmonary support (CPS) system. They were also therapeutically cooled to lower the patient’s metabolism and reduce oxygen demand. Dr. Revenaugh said none of the physicians involved in the case expected the patient to recover, but to their surprise the cardiac support helped reduce the load on the heart and the patient did recover.
“There is real value in these cases for using these devices instead of whipping the heart with dobutamine,” Dr. Revenaugh said.
VADs for Cath Lab Support
There are basically three types of VADs – intra-aortic balloon pumps (IABPs); pumps using catheters that can be placed in the left ventricle without surgical intervention; and pumps using surgically implanted cannulas.
IABPs have the advantages being easy to use, relatively inexpensive, low complication rates, and they are a mature technology that has been around since the 1970s, said Tom Piemonte, M.D., FACC, FSCA, director, interventional cardiac medicine, Lahey Clinic, Burlington, Mass. The clinic tested the TandemHeart, Impella and IABPs in a prophylactic manner for patients with progressive heart failure. He said the downsides to IABPs include no benefit in LV function and they do not actively unload the heart.
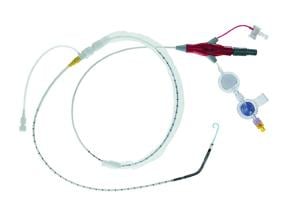
The Abiomed’s Impella 2.5 uses a 13 Fr. introducer sheath and its pig-tail type cannula is guided through the vasculature into the left ventricle. It is designed for quick deployment. The Impella is a little more expensive and a little more challenging to use compared to an IABP, said Howard A. Cohen, M.D., FSAI, director, division of cardiac interventions, Lenox Hill Heart and Vascular Institute, N.Y. However, Dr. Naidu said the Impella also provides much more flow than an IABP, because IABPs push blood in both directions in the aorta. He said this flow back toward the heart can also be a big issue if the patient has aortic valve regurgitation.
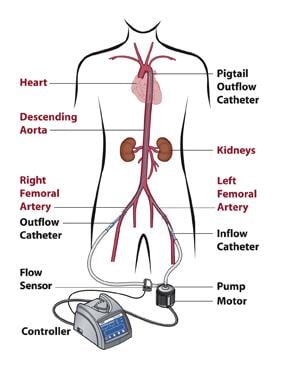
Dr. Cohen said CardiacAssist’s TandemHeart PTVA System is more expensive and more difficult to install than the Impella. Dr. Piemonte said he has seen the device offer benefits to patients in cardiogenic shock, but there can be vascular complications and implanting the device requires a 17 Fr. or larger transeptal puncture. He added the left atrium cannula can sometimes be unstable, and there are complex nursing requirements using the TandemHeart.
Intermountain uses the TandemHeart, Impella 2.5 and a CPS system for patients in cardiogenic shock and stage III and IV heart failure. Dr. Revenaugh added that the TandemHeart is also used for cardiac support during ventricular tachycardia ablations.
Better Perfusion in AMI Patients
Patients with acute myocardial infarction (AMI) face a lack of oxygen to the myocardium, which in turn lowers ejection fraction and blood/oxygen supply to the rest of the body. VADs can quickly increase blood flow without straining an ischemic heart.
Dr. Naidu uses the Impella 2.5 and says it can be used as a bridge to recovery in AMI patients. From his experience it is easier to use and quicker to deploy than most VADs or IABPs.
“The quicker you get it in, the better off they will be in preventing organ failure,” Dr. Naidu said.
Dr. Cohen said his experience shows VADs help reduce infarct size. He primarily uses VADs to support patients with sole remaining vessel and left ventricle dysfunction. However, he said VADs are also frequently used in his cath lab when treating high-risk lesions and when a large amount of the myocardium is ischemic.
“The more support you can give earlier on reduces the size of the infarct,” Dr. Cohen said.
Dr. Cohen said VADs gave him an edge when a woman with three-vessel disease arrived in his cath lab and was clinically dead. He used a VAD to keep her blood flowing while he performed PCI and she survived.
Dr. Naidu is involved with the PROTECT II clinical trial, which examines Abiomed’s Impella device in AMI and high-risk PCI patients for support up to five days. The trial is also comparing the IMPELLA LP 2.5 system to IABPs in preventing intra and post procedural major adverse events.
“I think the primary use will be to assist in the cath lab,” Dr. Naidu said. “Initially that is where about 80 percent of the (VAD) use is now.”



 August 14, 2023
August 14, 2023 









