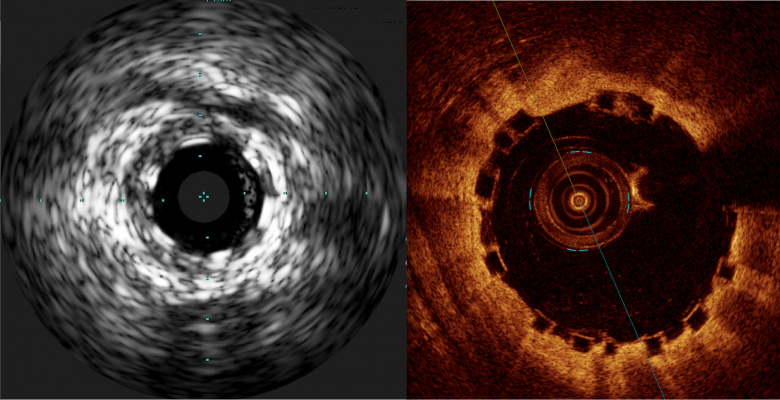
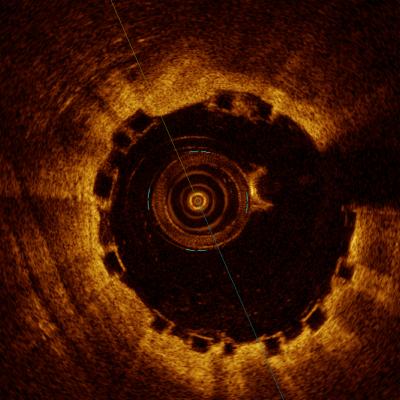
A comparison of how the Abbott Absorb BVS appears with intravascular ultrasound (IVUS) on the left, and optical coherence tomography (OCT) of the right. The stent is difficult to visualize and sizing is critical, so both modalities can help in bioresorbable stent measurements and to confirm stent apposition. Left image from the Volcano IVUS system and the right image from St. Jude Medical's OCT system.
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of interventional cardiology. These novel devices follow the successive evolutions of angioplasty, bare metal stents (BMS) and drug-eluting stents (DES), each of which led to a marked improvement in clinical outcomes for patients suffering from coronary artery disease (CAD). The most exciting aspect of BRS is preliminary data shows the vessel is healing, and histologically looks nearly normal approximately three years after scaffold implantation. CAD accounts for almost 60 percent of the 610,000 heart disease-related U.S. deaths annually. Improvements in stent technology and percutaneous coronary intervention (PCI) in general have had profound impacts on reducing these statistics, but much remains to be done and many interventional cardiologists are hopeful BRS can pave the way.
Benefits of Bioabsorbable Stents
Similar to earlier stents, successful PCI with BRS results in improvement in blood flow immediately following PCI. After performing this primary scaffold support function, BRS then reabsorb to help restore the vessel to its natural state over time, something that cannot be achieved with metal stents. Without a permanent metal structure left behind, natural and important functions of the artery vessel such as vasomotion and normalization of shear stress patterns resume. This leads to a big improvement over BMS and DES. As well, problems such as late-term thrombosis, bleeding, restenosis and others can be reduced or avoided altogether with BRS. Last, the absence of a permanent metal stent facilitates the ability to conduct future interventions, such as treatment of side branches or even coronary bypass grafts, as necessary.
Implementation in the U.S. and Clinical Justification
The Absorb GT1 Bioresorbable Vascular Scaffold (BVS) System, manufactured by Abbott, received regulatory approval in July 2016 from the U.S. Food and Drug Administration (FDA) as the first BRS to be marketed in the United States. Data supporting the approval was collected from the ongoing, randomized ABSORB clinical trial. The remarkable thing about the ABSORB data is that in the short term, the BRS is at least as effective as the current, best-generation DES.
Read about the FDA approval of the Absorb GT1 Bioresorbable Vascular Scaffold (BVS).
Challenges With BRS
Compared to DES, the inherent structure of BRS poses challenges in PCI. Metallic DES have a radio-opaque metal “cage” that is relatively easy to see under fluoroscopy and angiography. As well, its metal cage can extend significantly beyond its rated expansion diameter. For example, if the vessel and consequently the stent are not sized correctly (undersized in particular), the stent can be further stretched to reach full expansion and apposition against the vessel wall. This is the goal for all stent implantations.
In contrast, the BRS is made of a polymer, resulting in radiolucency, larger struts (compared to metallic BMS or DES) and limited expandability. These physical characteristics hamper delivery of the scaffold and suggest a more deliberate planning strategy is needed to mitigate these challenges.
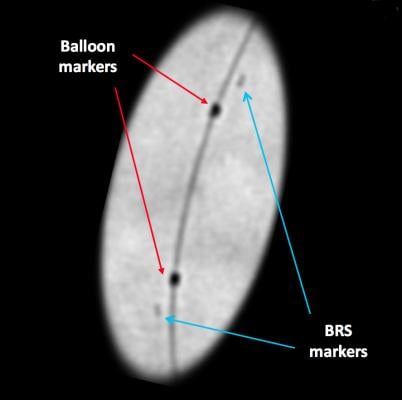
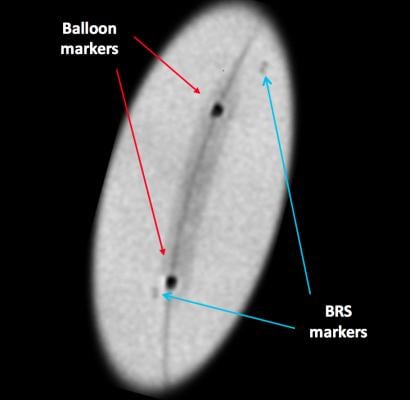
Since the scaffold is radiolucent, it is very difficult to see the BRS under fluoroscopy or angiography. To ensure the BRS location can be seen under X-ray during and after deployment (and after resorption), four radio-opaque markers are embedded near both edges of the scaffold (and on the accompanying Abbott balloon). These markers are small and may still be difficult to see under X-ray, although enhanced angiography can help improve visualization of the scaffold.
The strut geometry of the Absorb BVS is similar to the Xience DES; however, Absorb’s strut thickness of 150 microns is almost 50 percent larger than Xience (83 microns). Since inception, stent design has been a tradeoff between radial strength and strut thickness. Generally, the thicker the struts the higher the radial strength of the stent. However, since the polymer is weaker than metal, initial BRS designs have had to use larger struts to ensure delivery of adequate force and full expansion without affecting scaffold integrity. Other complications related to larger BRS struts include limited maneuverability within some vessels and crossing issues with proximal or bifurcated stents. Last, the polymer structure is relatively inelastic and BRS’s ability to fully expand is more limited (<0.5 mm) compared to BMS or DES (about 1.5 mm).
To overcome these challenges, thorough planning and a precise delivery technique is needed, beyond that required for BMS or DES. Key to the planning is the selection of the right imaging technologies to enable physicians a clear and full assessment of the proximal portion of the vessel, through which the BRS has to be advanced, and of the diseased area in order to prepare the vessel and size and implant the scaffold properly.
BRS Implant Procedures
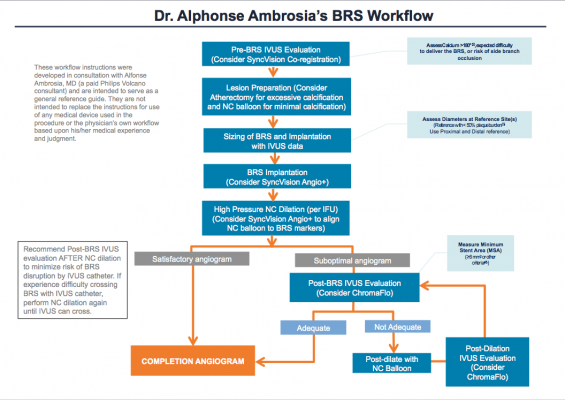
While participating in the clinical trial and analyzing the findings of ABSORB II and III, I recognized that part of the solution in overcoming the above challenges with BRS was to try and standardize the various BRS protocols currently being used. Certainly there have been issues with implantation based on the initial high rates of BRS thrombosis (versus DES), especially in the smaller vessels. I realized BRS is a different animal requiring a different set of delivery procedures that could not just be extrapolated from our DES experience. As well, many physicians were using this BRS device for the first time during the ABSORB trials. So I have taken my interventional experience with BRS, including lessons learned from the European experience, and present here a recommended workflow for BRS implantation. In this protocol, I recommend the use of intravascular ultrasound (IVUS) and SyncVision as the primary imaging tools to provide the right information at the right time to properly plan, guide and confirm BRS cases.
Assessment and Preparation for Bioresorbable Stents
Critical to this step is the proper evaluation of the type and extent of plaque and quantitatively determining vessel size to avoid problems achieving adequate expansion and apposition. The main benefit of IVUS is that it provides clear, detailed information on plaque burden, lesion morphology, and the location of key anatomical landmarks and reference segments. This information enables the interventionalist to develop their treatment strategy prior to implanting the BRS. Before I continue however, a quick review of current imaging devices is germane.
Mainstream PCI imaging includes angiography, IVUS and optical coherence tomography (OCT), each with relative strengths and weaknesses. None of them can individually provide the optimal complete picture for PCI procedures involving BRS, which makes sense given the different technological underpinnings of the three diagnostics. For example, the angiogram provides a good overall assessment of the coronary tree and suspected problem areas, but is not the best option for determining the detailed measurements of the diseased vessel necessary for successful completion of subsequent protocol steps. IVUS can penetrate deep into plaque (8-9 mm) to assess its morphology, true lumen and vessel remodeling, whereas OCT can only see approximately 2-3 mm deep, leaving the interventionalist guessing on the extent of the plaque. More compelling is IVUS’ ability to identify the calcified or fibrous plaques, which is one of the toughest complicating factors in interventional cardiology. OCT has far greater surface resolution than both angiography and IVUS and can be superior in post-deployment assessment, yet neither OCT or IVUS can see the big picture of what the angiogram sees. Last, both OCT and angiography require contrast injections to be able to see important aspects of the disease, whereas IVUS does not. These result in IVUS being more agile with rapid imaging production to base PCI decisions on. As I am familiar with both OCT and IVUS, I also believe IVUS is the more intuitive of the two, and more widely used in the United States. OCT presents a larger dataset of information, but it can be difficult to interpret for new users.
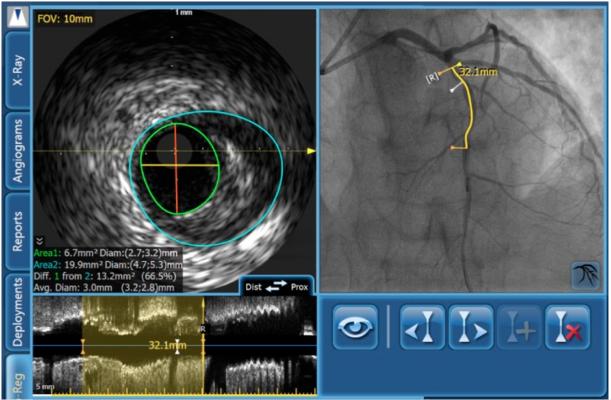
I continue with a thorough evaluation of the diseased area utilizing Philips Volcano’s SyncVision co-registration. By co-registering angiography with IVUS on one correlated screen, SyncVision tells physicians where they are on the arterial tree, including potential problem areas with the enhanced angiography. This enables the interventionalist to focus in on the areas of suspected disease with the IVUS component. IVUS visualizes the vessel, including its size, plaque burden, morphology, and other anatomical features and reference segments. For complex lesions with calcium buildup on more than 180 degrees of vessel circumference, consider a back-up plan. This might include extra support, such as a stronger guidewire or buddy wire, and a guideliner or other guide extension device prepped and ready to go if needed.
For the arteries with >180 degrees of calcium buildup, I want to prepare the calcified topography utilizing atherectomy. Although the safety and effectiveness of atherectomy has not been established with the Absorb GT1 BVS device, my clinical experience with atherectomy in BMS and DES cases has established it as an effective way of plaque modification to enhance delivery success of what would otherwise be a difficult implantation. Then I pre-dilate a non-compliant (NC) balloon and assess if the BRS is suitable in this case. For especially tortuous, complex lesions, defaulting to a more rugged DES may result in the best outcome. Given the BRS strut thickness and limited number of scaffold sizes available, intervention of vessels smaller than 2.5 mm on the distal end and/or larger than 3.75 mm on the proximal end should be avoided. For vessels with calcium burden less than 180 degrees in circumference, I will likely forego atherectomy, pre-dilate with an NC balloon or a scoring or cutting balloon and, if there is little residual stenosis, move to choosing the stent.
Read the article “Questions Remain on Future of Bioresorbable Stents.”
How to Choose Bioresorbable Stent Sizes
Due to its limited extendibility, correct sizing of the BRS is more critical to successful intervention than it is for metal stents. The margins for error are smaller and tolerances are tighter with BRS, requiring more precise planning and meticulous deployment. IVUS provides the accurate measurements necessary for the physician to decide on the proper stent size for a given lesion(s). This may reduce the need for excessive post-dilation to correct malapposition, geographic miss or other size-related problems. Use of SyncVision with its IVUS component to obtain accurate measurements is crucial, especially for vessels near the 2.5 mm limit.
With IVUS data, there are many options available to size the BRS. I recommend using the distal reference diameter as I am most concerned with distal dissections; however, I also check that the reference diameter works for the proximal reference as well. With only three sizes of BRS scaffolds being tested in the ABSORB trials — 2.5, 3 and 3.5 mm — the selection is made based on the closest scaffold size to the distal reference diameter. The diameter may be measured as lumen, mid-wall or vessel depending on the vessel plaque morphology. As a rule, I use the lumen diameter of the least diseased segments proximally and distally determined by IVUS. If the proximal and/or distal segment is significantly diseased, I can also use the media-to-media measurement at the lesion site if it is visible. I then select the stent diameter that is closest to the distal lumen diameter or media-to-media diameter at the lesion that I can dilate proximally without overexpanding the scaffold.
Regarding length, SyncVision indicates very accurately where the plaque burden begins and ends. Accordingly, I select the scaffold length that best matches the plaque ends. In this manner, the lesion is fully covered while mitigating edge dissection risk.
Deploying Bioresorbable Stents
Once the assessment is complete, the lesion prepared, and pre-dilation and sizing finished, I deploy the scaffold. I am looking for as close to a 1:1 ratio of vessel and scaffold size alignment as possible, prior to deployment. For this operation, I use a special feature of SyncVision called Angio+Device Detection to ensure accurate placement. Angio+Device Detection is a real-time image stabilization (from cardiac motion) and device enhancement that enables precise NC balloon/BRS/lesion alignment and delivery. Once the deployment balloon and scaffold are in position, the balloon is gradually inflated (2 ATMs every 5 seconds) up to its nominal pressure. I continue to slowly pressurize the balloon until full expansion is reached, somewhere between the nominal and rated burst pressure. When fully expanded, the pressure is held for 30 seconds to minimize acute recoil. Again using Angio+enhanced angiography, an evaluation of the deployment is conducted. If satisfactory (full expansion, full apposition, no edge dissection), the enhanced angiogram is recorded and I am almost finished. If not satisfactory, I move to the more detailed IVUS evaluation.
Post-deployment Evaluation of the BVS
In order to confirm full expansion, the use of IVUS is recommended as it gives the physician a more focused visualization of critical aspects of the scaffold and the diseased areas that might signal problems. If still concerned with edge dissection, I augment IVUS with a plug and play feature called ChromaFlo imaging. Seamlessly integrated with IVUS, ChromaFlo colors blood red on its images. This helps evaluate the lumen for any flow within the plaque distal to the stent, a signal for possible edge dissection. Scaffold apposition and lumen gain/loss are also more clearly depicted with ChromaFlo. I am looking for an optimum minimum stent area (MSA) based on the diameter of the stent implanted or similar measure. If the IVUS evaluation reveals good stent expansion and wall apposition without edge dissection and with adequate MSA, a completion angiogram is taken and I wrap things up. Otherwise, post-dilation is necessary.
Post-deployment Dilation of Bioresorbable Stents
The post-deployment, NC balloon dilation is crucial to successful implantation. You want to expand the balloon with adequate pressure to ensure the scaffold achieves full expansion, but not so high that you risk scaffold fracture. The NC balloon nominal diameter should be no greater than 0.5 mm higher than the nominal diameter of the scaffold.
Post-dilation with NC balloons can result in significant improvement of the MSA compared to compliant or semi-compliant balloons. Also, if there is a recalcitrant area of the lesion that is not expanding properly, a shorter NC balloon might be advisable to concentrate balloon pressure exclusively on the problem area. Compliant/semi-compliant balloons are tested in air and water, and their maximum atmosphere limitations are based on that milieu. Yet when deployed in an arteriosclerotic artery, the compliant balloon pressure is not able to expand the scaffold/plaque/vessel sufficiently. Worse, the compliant balloon increases in volume and diameter, which can lead to overexpansion and edge dissection. Use of NC balloons is highly recommended.
Dual Antiplatelet Therapy for Bioresorbable Stents
Last but not least, administration of dual antiplatelet therapy (DAPT) should be considered. Most clinical studies support at least one year of a combination of aspirin and P2Y platelet receptor inhibitors to reduce the risk of stent thrombosis following DES deployment. Preloading may be advisable. Although there has been a move toward reduced duration of DAPT therapy in newer-generation DES, at least 12 months of DAPT therapy is still advisable in BRS until longer-term data is available.
Advice to the Interventional Community
I believe the success of the BRS technology lies with the interventional cardiologist. In the near term, initial scaffold challenges such as its radiolucency, large strut size and brittle composition will require meticulous adherence to protocol and technique to overcome. Our recommended protocol outlined above is a good start, but anticipate the FDA to weigh in on standardized procedures for this nascent technology. My advice to colleagues is to focus on the upfront planning and assessment tasks such as selecting what imaging to use, choice of wires and catheters, evaluating the disease, etc. For those not confident with intravascular imaging, it would be prudent to get some experience with IVUS and SyncVision on DES you are delivering today. That way the transition to BRS will be much easier and successful and will not bring back memories of first-generation BMS.
Read the article “Robotics May Aid Implantation of Bioresorbable Stents.”
Editor’s note: Alphonse Ambrosia, D.O., is an interventional cardiologist at Banner Heart Hospital in Mesa, Ariz.


 November 14, 2025
November 14, 2025 









