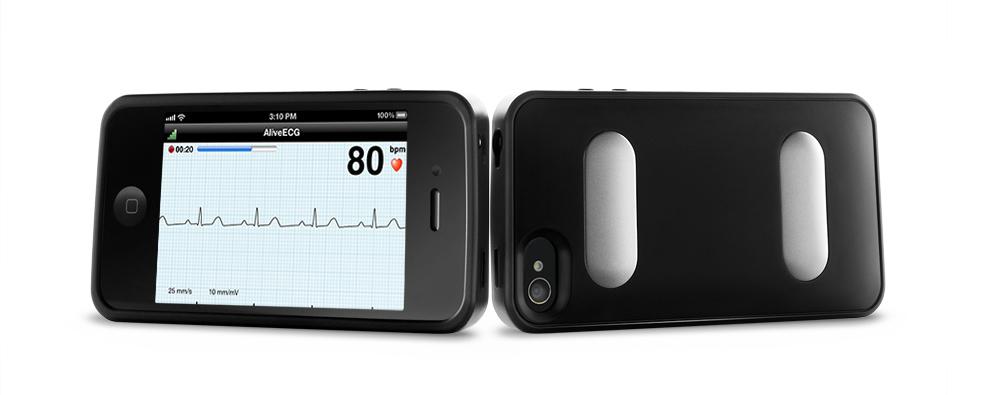
April 18, 2014 — AliveCor announced the online publication of study results of the SEARCH-AF study. The SEARCH-AF study, conducted in Australia, screened 1,000 customers over 65 years old for atrial fibrillation (AF) in 10 community pharmacies in suburban Sydney.
The AliveCor heart monitor was used to capture 30-60 second ECG (electrocardiogram) recordings and wirelessly transmit the recordings to study cardiologists. This simple method of remote ECG capture enabled study cardiologists to identify patients at risk of stroke because of unknown AF, and flag them for additional evaluation. New AF was identified in 1.5 percent of the people screened, all at high risk of stroke. Most of the people with newly discovered AF had no symptoms, and may never have sought medical advice.
This study highlights one application of mobile ECG technology that can help facilitate cost-effective preventative medical care. Additionally, the study found the incremental cost-effectiveness ratio was just over $4,000 for an increase of one quality adjusted life year (QALY) and just over $20,000 for preventing one stroke. If asymptomatic people with AF are detected in this way and given warfarin or other newer blood thinners, the risk of stroke can potentially be reduced by two thirds.
The study, “Feasibility and cost effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies,” appeared in the April 1 online issue of Thrombosis and Haemostasis and was led by Ben Freedman, M.D., professor of cardiology, Concord Hospital Department of Cardiology and Anzac Research Institute, Sydney Medical School, University of Sydney.
“Community screening using the AliveCor heart monitor in pharmacies has shown to be both feasible and cost-effective in helping physicians identify people with AF, the most common abnormal heart rhythm, which is responsible for a third of all strokes,” said Freedman. “In many cases AF is not known before a stroke, so screening for AF and treating with effective medications could make an impact on reducing the community burden of stroke.”
“We believe these findings have great significance for health providers and should become part of routine practice as this type of screening program is a cost-effective way to identify patients with asymptomatic atrial fibrillation, a condition that can lead to potentially life threatening complications,” said Euan Thomson, president and CEO of AliveCor.
The AliveCor heart monitor is the only U.S. Food and Drug Administration (FDA) cleared mobile ECG recorder that supports both iPhone and Android smartphones. It records, displays (when prescribed or used under the care of a physician), stores and transfers single-channel ECG rhythms wirelessly, using the free AliveECG app. With secure storage in the cloud, users can access their data confidentially anytime, anywhere, and can grant access to their physicians. The heart monitor is available for purchase in the United States by health professionals and consumers.
For more information: www.alivecor.com


 July 22, 2025
July 22, 2025 



