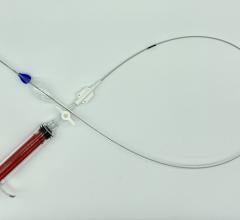
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR), a new startup company has developed a transfemoral combined access and closure system to accomodate these larger sized percutaneous devices and arteriotomies. Introduced in sessions at the 2012 American College of Cardiology (ACC) and EuroPCR, the VasoStitch system is designed to reduce procedural complexity and eliminate the need for open surgical cut-downs and surgical closure of the vessel.
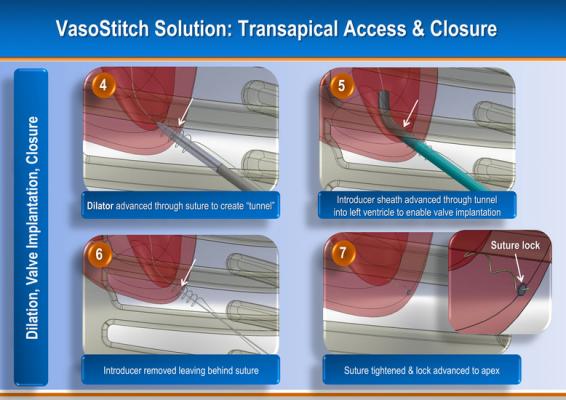
The company is also developing a similar transapical TAVR access and closure device to eliminate the need to sew surgical purse-string sutures at the apex of the heart prior to introducer placement. This may significanty shorten and simplify transapical valve replacement procedures.
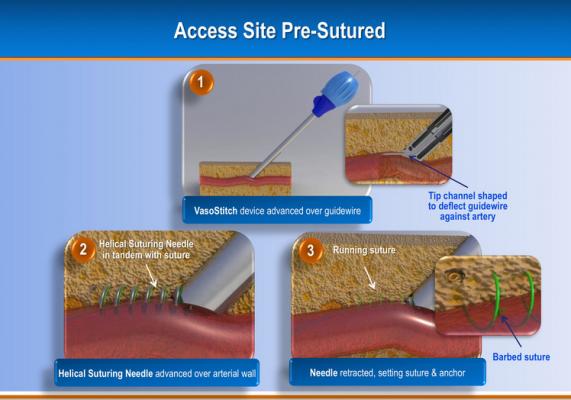
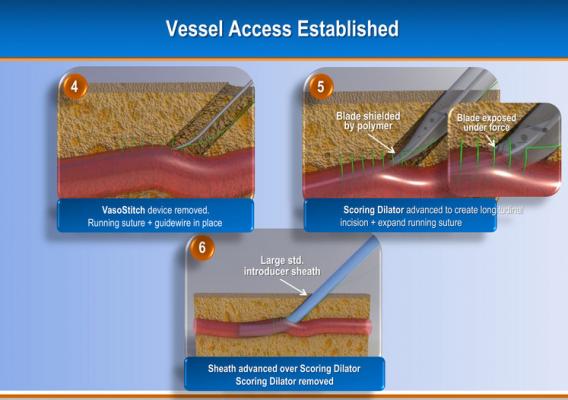
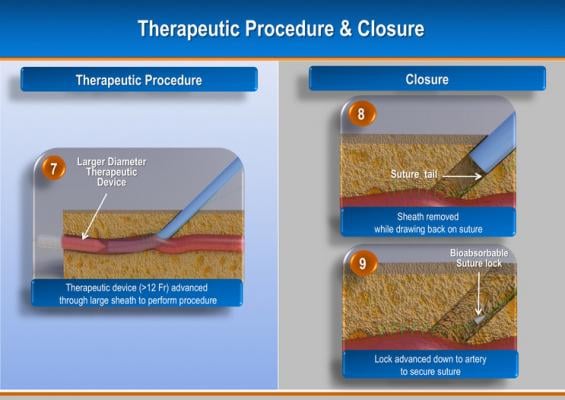
The system uses a helical, corkscrew-shaped, barbed suturing needle that is advanced through the vessel wall and surrounding tissue prior to vessel access. Access is accomplished though the center of the suture needle coil. When the procedure is complete, a suture tail is pulled to close the vessel and activate a bioresorbable suture lock.
“At the present time, surgical means of arterial or cardiac access-and-closure for large-diameter, catheter-based interventions such as TAVI adds about 60 minutes to an already lengthy and elaborate procedure. As a result, a device that can duplicate the current ‘gold standard’ of surgical access and repair — but in a quicker and simpler, nonsurgical procedure — will surely stimulate the adoption of large-diameter cardiovascular and endovascular therapies, as well as improve the efficiency of patient management,” said David W. J. Smith, president and CEO of Danville, Calif.-based VasoStitch. “We are moving quickly toward our first-in-human milestone.”
For more information: www.vasostitch.com


 September 18, 2025
September 18, 2025